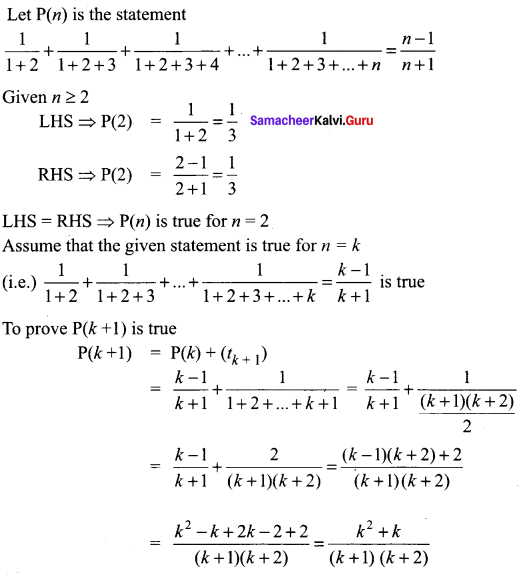
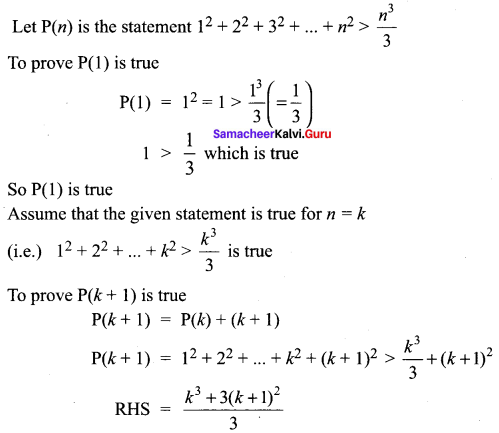
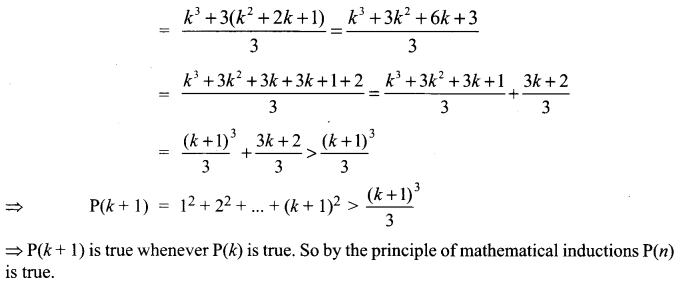
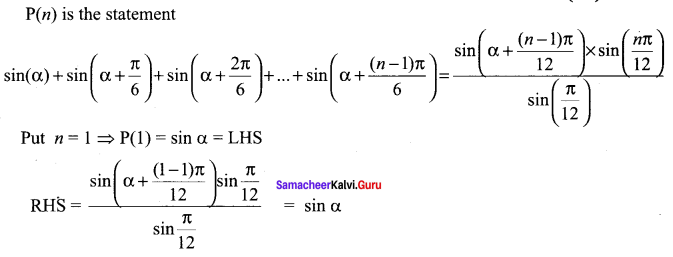
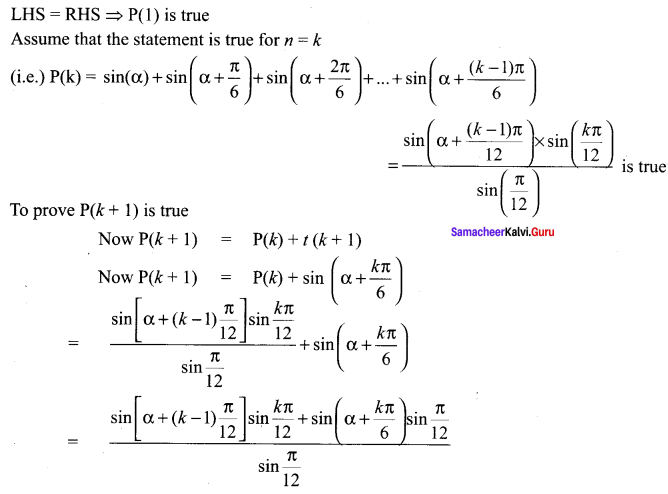
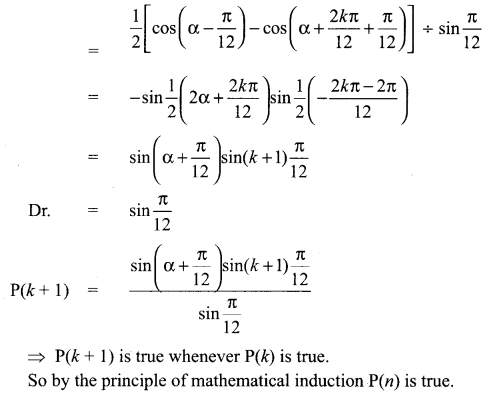
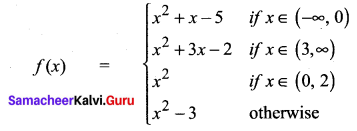
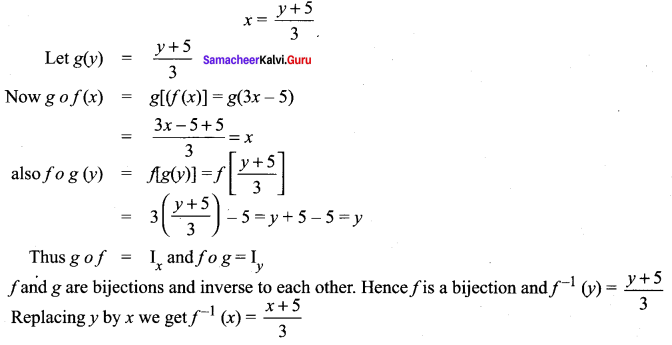
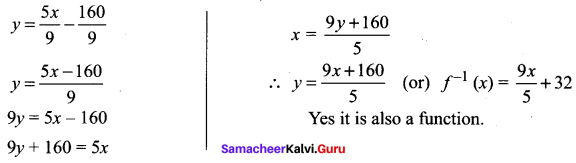
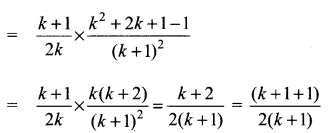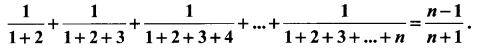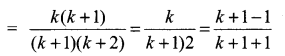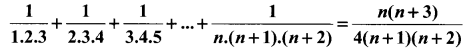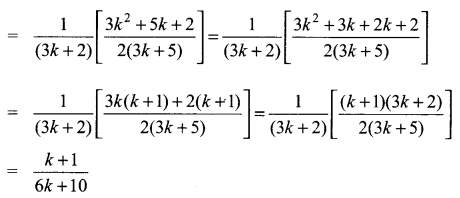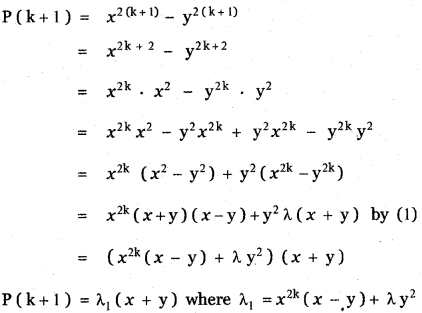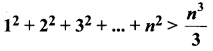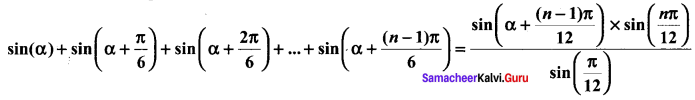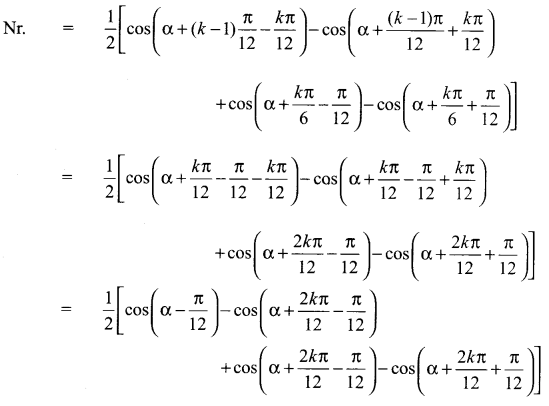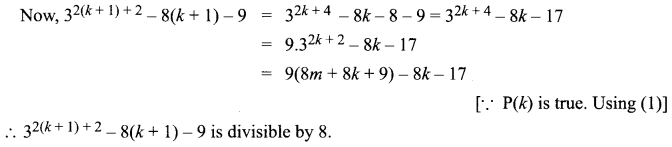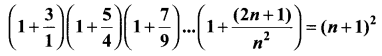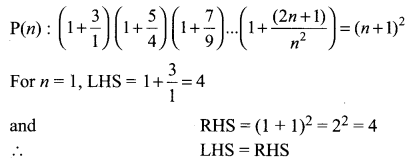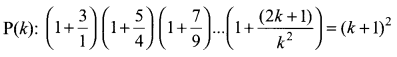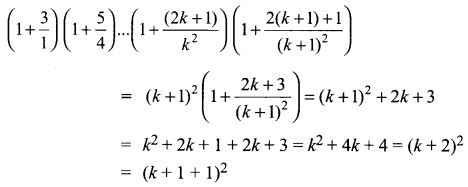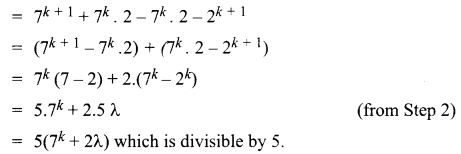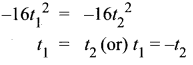You can Download Samacheer Kalvi 10th Science Book Solutions Guide Pdf, Tamilnadu State Board help you to revise the complete Syllabus and score more marks in your examinations.
Tamilnadu Samacheer Kalvi 10th Science Solutions Chapter 5 Acoustics
Samacheer Kalvi 10th Science Acoustics Textual Solved Problems
Question 1.
At what temperature will the velocity of sound in the air be double the velocity of sound in air at 0° C?
Solution:
Let T° C be the required temperature. Let v1 and v2 be the velocity of sound at temperatures T1K and T2K respectively.
T1 = 273K (0°C) and T2 = (T°C + 273)K
\(\frac{v_{2}}{v_{1}}=\sqrt{\frac{\mathrm{T}_{2}}{\mathrm{T}_{1}}}=\sqrt{\frac{273+\mathrm{T}}{273}}=2\)
Here, it is given that, v2/v1 = 2
So \(\frac{273+\mathrm{T}}{273}=4\)
T = (273 × 4) – 273 = 819° C.
Question 2.
A source producing a sound of frequency 90 Hz is approaching a stationary listener with a speed equal to (1 / 10) of the speed of sound. What will be the frequency heard by the listener?
Solution:
When the source is moving towards the stationary listener, the expression for apparent frequency is
\(n^{\prime}=\left(\frac{v}{v-v_{s}}\right) n\)
\(\begin{array}{l}{=\left(\frac{v}{v-\left(\frac{1}{10}\right) v}\right) n=\left(\frac{10}{9}\right) n} \\ {=\left(\frac{10}{9}\right) \times 90=100 \mathrm{Hz}}\end{array}\)
Question 3.
A source producing a sound of frequency 500 Hz is moving towards a listener with a velocity of 30 ms-1. The speed of the sound is 330 ms-1. What will be the frequency heard by the listener?
Solution:
When the source is moving towards the stationary listener, the expression for apparent frequency is
n’ = \(\left(\frac{v}{v-v_{\mathrm{s}}}\right) n\)
= \(\left(\frac{330}{330-30}\right) \times 500\)
= 550 Hz.
Question 4.
A source of sound is moving with a velocity of 50 ms-1 towards a stationary listener. The listener measures the frequency of the source as 1000 Hz. what will be the apparent frequency of the source when it is moving away from the listener after crossing him? (velocity of sound in the medium is 330 ms-1)
Solution:
When the source is moving towards the stationary listener, the expression for apparent frequency is
\(\begin{aligned} n^{\prime} &=\left(\frac{v}{v-v_{\mathrm{s}}}\right) n \\ 1000 &=\left(\frac{330}{330-50}\right) n \\ n &=\left(\frac{1000 \times 280}{330}\right) \end{aligned}\)
n = 848.48 Hz.
The actual frequency of the sound is 848.48 Hz. When the source is moving away from the stationary listener, the expression for apparent frequency is
\(\begin{aligned} n^{\prime} &=\left(\frac{v}{v+v_{\mathrm{s}}}\right) n \\ &=\left(\frac{330}{330+50}\right) \times 848.48 \end{aligned}\)
= 736.84 Hz.

Question 5.
A source and listener are both moving towards each other with a speed v/10 where v is the speed of sound. If the frequency of the note emitted by the source is f, what will be the frequency heard by the listener?
Solution:
When source and listener are both moving towards each other, the apparent frequency is
\(\begin{aligned} n^{\prime} &=\left(\frac{v+v_{l}}{v-v_{s}}\right) n \\ n^{\prime} &=\left(\frac{v+\frac{v}{10}}{v-\frac{v}{10}}\right) n \\ n^{\prime} &=\frac{11}{9} \cdot f \\ &=1.22 f \end{aligned}\)
Question 6.
At what speed should a source of sound move away from a stationary observer so that the observer finds the apparent frequency equal to half of the original frequency?
Solution:
When the source is moving away from the stationary listener, the expression for the apparent frequency is
\(\begin{array}{l}{n^{\prime}=\left(\frac{v}{v+v_{\mathrm{s}}}\right) n} \\ {\frac{n}{2}=\left(\frac{v}{v+v_{\mathrm{s}}}\right) n} \\ {v_{\mathrm{s}}=v}\end{array}\)
Samacheer Kalvi 10th Science Acoustics Textual Evaluation
I. Choose the best Answer
Question 1.
When a sound wave travels through air, the air particles _____.
(a) vibrate along the direction of the wave motion
(b) vibrate but not in any fixed direction
(c) vibrate perpendicular to the direction of the wave motion
(d) do not vibrate.
Answer:
(a) vibrate along the direction of the wave motion
Question 2.
Velocity of sound in a gaseous medium is 330 ms-1. If the pressure is increased by 4 times without causing a change in the temperature,
the velocity of sound in the gas is:
(a) 330 ms-1
(b) 660 ms1
(c) 156 ms-1
(d) 990 ms-1
Answer:
(a) 330 ms-1
Question 3.
The frequency, which is audible to the human ear is _____.
(a) 50 kHz
(b) 20 kHz
(c) 15000 kHz
(d) 10000 kHz.
Answer:
(b) 20 kHz
Hint: Audible frequency hearing the human ear range between 20 Hz to 20 kHz
Question 4.
The velocity of sound in air at a particular temperature is 330 ms-1. What will be its value when temperature is doubled and the pressure is halved?
(a) 330 ms-1
(b) 165 ms-1
(c) 330 × √2 ms-1
(d) 320 × √2 ms-1
Answer:
(a) 330 ms-1
Question 5.
If a sound wave travels with a frequency of 1.25 × 104 Hz at 344 ms-1, the wavelength will be _____.
(a) 27.52 m
(b) 275.2 m
(c) 0.02752 m
(d) 2.752 m.
Answer:
(c) 0.02752 m
Hint: Velocity of sound = frequency × wavelength
v = nλ
\(\lambda=\frac{v}{n}=\frac{344}{1.24 \times 10^{4}}\)
λ = 0.02752 m.

Question 6.
The sound waves are reflected from an obstacle into the same medium from which they were incident. Which of the following changes?
(a) speed
(b) frequency
(c) wavelength
(d) none of these.
Answer:
(d) none of these.
Hint: If there is the same medium of a reflected obstacle is used, there is no change in speed, frequency and wavelength of the sound.
Question 7.
Velocity of sound in the atmosphere of a planet is 500 ms-1. The minimum distance between the sources of sound and the obstacle to hear the echo, in point Is is:
(a) 17 m
(b) 20 m
(c) 25 m
(d) 50 m
Answer:
(c) 25 m
II. Fill in the blanks
Question 1.
The rapid back and forth motion of a particle about its mean position is called _____.
Answer:
vibrations.
Question 2.
If the energy in a longitudinal wave travels from south to north, the particles of the medium would be vibrating in _____.
Answer:
both north and south.
Question 3.
A whistle giving out a sound of frequency 450 Hz, approaches a stationary observer at a speed of 33 ms-1. The frequency heard by the observer is (speed of sound = 330 ms-1) ______.
Answer:
Data:
v = 330 ms-1
vS = 33 ms-1
n = 450 Hz
n’ =?
Apparent frequency
\(\begin{aligned} n^{\prime} &=\left(\frac{v}{v-v_{\mathrm{S}}}\right) n \\ &=\left(\frac{330}{330-33}\right) \times 450=1.11 \times 450 \end{aligned}\)
n’ = 500 Hz.
Question 4.
A source of sound is travelling with a velocity 40 km/h towards an observer and emits a sound of frequency 2000 Hz. If the velocity of sound is 1220 km/h, then the apparent frequency heard by the observer is _____.
Answer:
2068 Hz
Hint: Apparent frequency heard by the observer.
Data:
v = 1220 km/h (or) 339 ms-1
vS = 40 km/h (or) 11.1 ms-1
n = 2000 Hz
\(\begin{aligned} n^{\prime} &=\left(\frac{v}{v-v_{\mathrm{s}}}\right) n \\ &=\left(\frac{339}{339-11.1}\right) \times 2000 \end{aligned}\)
= 1.03385 × 2000
n’ = 2068 Hz.
III. True or False. If false give the reason
Question 1.
Sound can travel through solids, gases, liquids and even vacuum.
Answer:
False.
Reason: Sound can not travel in a vacuum. This is because sound waves are vibrating waves. In vacuum, where there are no atoms or molecules to vibrate.
Question 2.
Waves created by Earth Quake are Infrasonic.
Answer:
True.

Question 3.
The velocity of sound is independent of temperature.
Answer:
False.
Reason: For an ideal gas the velocity of sound depends on its temperature and is independent of gas pressure.
Question 4.
The velocity of sound is high in gases than liquids.
Answer:
False.
Reason: Velocity of a sound wave is maximum in solids because they are more elastic in nature than liquids and gases. Since gases are least elastic in nature, the velocity of sound is the least in a gaseous medium.
So VS > VL > VG.
IV. Match the following
Question 1.
| 1. Infrasonic |
(a) Compressions |
| 2. Echo |
(b) 22 kHz |
| 3. Ultrasonic |
(c) 10 Hz |
| 4. High-pressure region |
(d) Ultrasonography |
Answer:
1. (c) 10 Hz
2. (d) Ultrasonography
3. (b) 22 kHz
4. (a) Compressions
V. Assertion and Reason Type Questions
Mark the correct choice as
(a) If both the assertion and the reason are true and the reason is the correct explanation of the assertion.
(b) If both the assertion and the reason are true but the reason is not the correct explanation of the assertion.
(c) The assertion is true, but the reason is false.
(d) The assertion is false, but the reason is true.
Question 1.
Assertion: The change in air pressure affects the speed of sound.
Reason: The speed of sound in a gas is proportional to the square of the pressure
Answer:
(d) The assertion is false, but the reason is true.
Question 2.
Assertion: Sound travels faster in solids than in gases.
Reason: Solid posses a greater density than that of gases.
Answer:
(b) If both the assertion and the reason are true but the reason is not the correct explanation of the assertion.
VI. Answer very briefly
Question 1.
What is a longitudinal wave?
Answer:
If the particles of the medium vibrate along the direction of propagation of the wave.
Question 2.
What is the audible range of frequency?
Answer:
Audible range of frequency is from 20 Hz to 20000 Hz.
Question 3.
What is the minimum distance needed for an echo?
Answer:
- The minimum distance required to hear an echo is 1/20th part of the magnitude of the velocity of sound in air.
- If you consider the velocity of sound as 344 ms-1, the minimum distance required to hear an echo is 17.2 m.
Question 4.
What will be the frequency sound having 0.20 m as its wavelength, when it travels with a speed of 331 m s-1?
Answer:
Speed v = 331 m/s
Let the wave length be λ = 0.2 m
Let the frequency be n
∴ Frequency n = \(\frac{v}{λ}\) = \(\frac{331}{0.2}\) = \(\frac{3310}{2}\)
∴ Frequency = 1655 Hz

Question 5.
Name three animals, which can hear ultrasonic vibrations.
Answer:
Mosquitos, bats and dogs are the three animals that can hear ultrasonic vibrations.
VII. Answer briefly
Question 1.
Why does sound travel faster on a rainy day than on a dry day?
Answer:
When humidity increases, the speed of sound increases. That is why you can hear sound from long distances clearly during rainy seasons.
Question 2.
Why does an empty vessel produce more sound than a filled one?
Answer:
In an empty vessel, multiple reflections of sound takes place. Hence more sound is produced in an empty vessel than tilled one.
Question 3.
Air temperature in the Rajasthan desert can reach 46°C. What is the velocity of sound in air at that temperature? (v0 = 331 ms-1)
Answer:
Velocity of sound, v0 = 331 ms-1
Air temperature, T = 46° C
Velocity of sound in air temperature vT = (v0 + 0.61T) ms-1
= 331 + (0.61 × 46)
= 331 + 28.06
vT = 359.06 ms-1.
Question 4.
Explain why the ceilings of concert halls are curved.
Answer:
The ceilings of concert halls are curved so that multiple reflections of sound waves can take place.
The parabolic surfaces are used to focus the sound at a particular point. Hence sound will be louder.
Question 5.
Mention two cases in which there is no Doppler effect in sound?
Answer:
- When source (S) and listener (L) both are at rest.
- When S and L move in such a way that distance between them remains constant.
- When source S and L are moving in mutually perpendicular directions.
- If the source is situated at the centre of the circle along which the listener is moving.
VIII. Problem Corner
Question 1.
A sound wave has a frequency of 200 Hz and a speed of 400 ms-1 in a medium. Find the wavelength of the sound wave.
Solution:
A sound wave of frequency, n = 200 Hz
Speed, v = 400 ms-1
The wavelength of the sound wave, λ = \(\frac{v}{n}\)
λ = \(\frac{400}{200}\) = 2 m.
Question 2.
The thunder of cloud is heard 9.8 seconds later than the flash of lightning. If the speed of sound in air is 330 ms-1, what will be the height of the cloud?
Answer:
Speed of sound in air v = 330 m/s
Time to hear thunder t = 9.8 s
∴ Height of the cloud = v × t
= 330 × 9.8
= 3234 m.
Question 3.
A person who is sitting at a distance of 400 m from a source of sound is listening to a sound of 600 Hz. Find the time period between successive compressions from the source?
Solution:
Frequency = \(\frac{1}{\text { Time period }}\)
Time period = \(\frac{1}{\text { Frequency }}=\frac{1}{600}\)
T = 0.00167 seconds
The time period between successive compressions from the source is 0.00167 seconds.
Question 4.
An ultrasonic wave is sent from a ship towards the bottom of the sea. It is found that the time interval between the transmission and reception of the wave is 1.6 seconds. What is the depth of the sea, if the velocity of sound in the seawater is 1400 ms-1?
Solution:
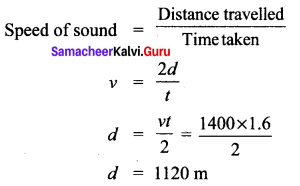
The depth of the sea is 1120 m.
Question 5.
A man is standing between two vertical walls 680 m apart. He claps his hands and hears two distinct echoes after 0.9 seconds and 1.1 seconds respectively. What is the speed of sound in the air?
Solution:
Given data is
d = 680 m
t1 = 0.9 s
t2 = 1.1 s
v = ?
\(\text { Velocity }=\frac{\text { Distance travelled by sound }}{\text { Time taken }}\)
\(v=\frac{2 d}{t}\)
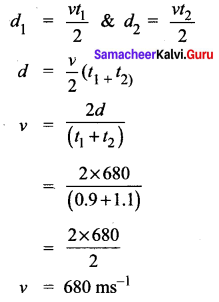
Question 6.
Two observers are stationed in two boats 4.5 km apart. A sound signal sent by one, under water, reaches the other after 3 seconds. What is the speed of sound in the water?
Answer:
Distance of boats d = 4.5 × 103 m
= 4500 m.
Time t = 3 s
Speed of sound in water = \(\frac{d}{t}\)
= \(\frac{4500}{3}\)
= 1500 m/s
Question 7.
A strong sound signal is sent from a ship towards the bottom of the sea. It is received back after 1 s What is the depth of sea given that the speed of sound in water 1450 ms-1?
Solution:
Velocity of sound in water \(=\frac{\text { Distance travelled by sound }}{\text { Time taken }}\)
Depth of the sea, d = velocity × time
\(d=\frac{v t}{2}=\frac{1450 \times 1}{2}\) = 725 m.
IX. Answer in Detail
Question 1.
What are the factors that affect the speed of sound in gases?
Answer:
In the case of gases, the following factors affect the velocity of sound waves.
Effect of density : The velocity of sound in a gas is inversely proportional to the square root of the density of the gas. Hence, the velocity decreases as the density of the gas increases.
v ∝\(\sqrt{\frac{1}{d}}\)
Effect of temperature : The velocity of sound in a gas is directly proportional to the square root of its temperature. The velocity of sound in a gas increases with the increase in temperature v ∝ √T. Velocity at temperature T is given by the following equation:
Vr = (vo + 0.61 T)ms-1.
Here, vo is the velocity of sound in the gas at 0° C. For air, vo = 331 ms-1. Hence, the velocity of sound changes by 0.61 ms-1 when the temperature changes by one degree Celsius.
Effect of relative humidity : When humidity increases, the speed of sound increases. That is why you can hear sound from long distances clearly during rainy seasons.
Question 2.
What is mean by the reflection of sound? Explain.
Answer:
Reflection of Sound:
The bouncing of sound waves from the interface between two media is termed as the reflection of sound.
(a) Reflection at the boundary of a rarer medium
- Consider a wave travelling in a solid medium striking on the interface between the solid and the air. The compression exerts a force F on the surface of the rarer medium.
- As a rarer medium has smaller resistance for any deformation, the surface of separation is pushed backwards.
- As the particles of the rarer medium are free to move, a rarefaction is produced at the interface. Thus, compression is reflected as rarefaction and a rarefaction travels from right to left.
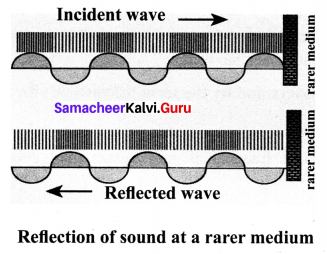
(b) Reflection at the boundary of a denser medium
- A longitudinal wave travels in a medium in the form of compressions and rarefactions. Suppose a compression travelling in the air from left to right reaches a rigid wall.
- The compression exerts a force F on the rigid wall. In turn, the wall exerts an equal and
opposite reaction R = -F on the air molecules. This results in a compression near the rigid wall.
- Thus, a compression travelling towards the rigid wall is reflected back as a compression. That is the direction of compression is reversed.
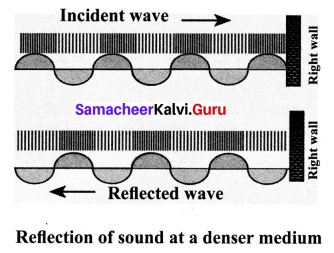
(c) Reflection at sound in curved surfaces
- When the sound waves are reflected from the curved surfaces, the intensity of the reflected waves is changed.
- When reflected from a convex surface, the reflected waves are diverged out and the intensity is decreased.
- When sound is reflected from a concave surface, the reflected waves are converged and focused at a point. So the intensity of reflected waves is concentrated at a point.
- Parabolic surfaces are used when it is required to focus the sound at a particular point. Hence, many halls are designed with parabolic reflecting surfaces.
- In elliptical surfaces, sound from one focus will always be reflected the other focus, no matter where it strikes the wall.
Question 3.
(a) What do you understand by the term ‘ultrasonic vibration’?
(b) State three uses of ultrasonic vibrations.
(c) Name three animals which can hear ultrasonic vibrations.
Answer:
(a) Ultrasonic vibrations are the vibration with frequency greater than 20 KHz. Human ear cannot detect the ultrasonic vibration.
(b) (i) Ultrasonic waves are used in ultrasonography.
(ii) It is used to get signal images of a developing embryo in the mother’s uterus.
(in) They are used to forecast about tsunami and earthquake.
(c) Certain creatures like dog, bats, dolphins and mosquito can detect the waves.
Question 4.
What is an echo?
(a) State two conditions necessary for hearing an echo.
(b) What are the medical applications of echo?
(c) How can you calculate the speed of sound using echo?
Answer:
Echo: An echo is the sound reproduced due to the reflection of the original sound from various rigid surfaces such as walls, ceilings, surfaces of mountains, etc.
(a) (i) The persistence of hearing for human ears is 0.1 second. This means that we can hear two sound waves clearly if the time interval between the two sounds is at least 0.1 s. Thus, the minimum time gap between the original sound and an echo must be 0.1 s.
(ii) The above criterion can be satisfied only when the distance between the source of the sound and the reflecting surface would satisfy the following equation:
\(\begin{aligned} \text { Velocity } &=\frac{\text { Distance travelled by sound }}{\text { Timetaken }} \\ v &=\frac{2 d}{t} \\ d &=\frac{v t}{2} \end{aligned}\)
Since, t = 0.1 second, then \(d=\frac{v \times 0.1}{2}=\frac{v}{20}\)
(b) (i) The principle of echo is used in obstetric ultrasonography, which is used to create real – time visual images of the developing embryo or fetus in the mother’s uterus.
(ii) This is a safe testing tool, as it does not use any harmful radiations.
(c) Apparatus required:
A source of sound pulses, a measuring tape, a sound receiver and a stopwatch.
Calculation of speed of sound:
The sound pulse emitted by the source travels a total distance of 2nd while travelling from the source to the wall and then back to the receiver.
The time taken for this has been observed to be ‘t’. Hence, the speed of the sound wave is given by:
\(\text { Speed of sound }=\frac{\text { Distance travelled }}{\text { Timetaken }}=\frac{2 d}{t}\).
X. HOT Questions
Question 1.
Suppose that a sound wave and a light wave have the same frequency, then which one has a longer wavelength?
(a) Sound
(b) Light
(c) both (a) and (b)
(d) data not sufficient.
Answer:
(b) light
Explanation: The light wave has a longer wavelength because it has much greater speed.

Question 2.
When sound is reflected from a distant object, an echo is produced. Let the distance between the reflecting surface and the source of sound remain the same. Do you hear an echo sound on a hotter day? Justify your answer.
Answer:
Echo of sound is heard with less velocity and intensity on a hotter day. Because the velocity of sound is directly proportional to temperature of air. On a hotter day temperature will be higher so velocity of sound will be changed by higher values.
Samacheer Kalvi 10th Science Acoustics Additional Questions
I. Choose the best answer
Question 1.
Light can travel in a vacuum but not sound because of ______.
(a) speed of sound is very slow than light.
(b) light waves are electromagnetic in nature.
(c) sound waves are electromagnetic in nature.
(d) light waves are not electromagnetic in nature.
Answer:
(a) speed of sound is very slow than light.
Hint: Light can travel in a vacuum because light waves are electromagnetic in nature.
Question 2.
In a longitudinal wave there is a state of maximum compression at a point at an instant. The frequency of wave is 50 Hz. After what time will the same point be in the state of maximum rarefaction:
(a) 50 s
(b) 25 s
(c) 0.01 s
(d) 0.002 s
Answer:
(c) 0.01 s
Question 3.
When sound travels from air to water, which parameter does not change?
(a) Wavelength
(b) Frequency
(c) Velocity
(d) Temperature.
Answer:
(b) Frequency
Hint: Frequency remains unchanged when sound travels from air to water.
Question 4.
When a wave passes from one medium to another, there is a change of:
(a) Wavelength and velocity
(b) Wavelength and frequency
(c) Velocity and frequency
(d) Velocity only
Answer:
(a) Wavelength and velocity
Question 5.
At what temperature, the speed of sound in the air will become double of its value at 27°C?
(a) 54° C
(b) 627° C
(c) 327° C
(d) 927° C.
Answer:
(d) 927° C.
Hint:
\(\frac{v_{1}}{v_{2}}=\sqrt{\frac{\mathrm{T}_{1}}{\mathrm{T}_{2}}} \Rightarrow \frac{v_{1}}{2 v_{1}}=\sqrt{\frac{\mathrm{T}_{1}}{\mathrm{T}_{2}}}\)
⇒ \(\frac{1}{4}=\frac{27+273}{\mathrm{T}_{2}}\)
⇒ T2 = 300 × 4 = 1200 K
⇒ T2 = 1200 – 273 = 927° C.

Question 6.
Sound waves from a point source are propagating in all directions. What will be the ratios of amplitude at a distance 9 m and 25 m from the source?
(a) 9 : 25
(b) 25 : 9
(c) 3 : 5
(d) 81 : 625
Answer:
(b) 25 : 9
II. Fill in the blanks:
Question 1.
Sound waves in a gas are ______.
Answer:
Longitudinal.
Question 2.
The velocity of sound is the largest in ______.
Answer:
Steel.
Question 3.
Hertz is the unit of ______.
Answer:
Frequency.
Question 4.
Sound travels in rocks in the form of ______.
Answer:
Both longitudinal and transverse elastic waves.
Question 5.
The velocity of sound varies directly as the square root of the ______.
Answer:
The absolute temperature of the gas.
Question 6.
Reflection of sound waves obey the ______.
Answer:
Laws of reflection.
Question 7.
RADAR is the short form of ______.
Answer:
Radio Detection and Ranging.

Question 8.
The abbreviation of SONAR is ______.
Answer:
Sound Navigation and Ranging.
III. Match the following
Question 1.
| Medium |
Speed of Sound (m/s) |
| (i) Copper |
(a) 343 |
| (ii) Water |
(b) 331 |
| (iii) Air (0° c) |
(c) 5010 |
| (iv) Air (at 20° c) |
(d) 1493 |
Answer:
(i) (c) 5010
(ii) (d) 1493
(iii) (b) 331
(iv) (a) 343.
Question 2.
| Waves |
Range |
| (i) Audible |
(a) 340 m/s |
| (ii) Sound |
(b) greater than 20kHz |
| (iii) Infrasonic |
(c) 20Hz – 20 kHz |
| (iv) Light |
(d) below 20 Hz |
| (v) Ultrasonic |
(e) 3 × 108 m/s |
Answer:
(i) (c) 20 Hz – 20 kHz
(ii) (a) 340 m/s
(iii) (d) below 20 Hz
(iv) (e) 3 × 108 m/s
(v) (b) greater than 20 kHz.
IV. Assertion and Reason Questions
Question 1.
Assertion: Sound wave cannot propagate through a vacuum but light can.
Reason: Sound wave cannot be polarised but light can.
(a) Both assertion and reason are true and the reason is the correct explanation of the assertion.
(b) Both assertion and reason are true but the reason is not the correct explanation of the assertion.
(c) Assertion is true but the reason is false.
(d) Both assertion and reason are false.
Answer:
(b) Both assertion and reason are true but the reason is not the correct explanation of the assertion.
Explanation: Sound waves, being mechanical waves, cannot travel through a vacuum. Light waves, electromagnetic waves, can travel through a vacuum.
Question 2.
Assertion: The velocity of sound increases with increase in humidity.
Reason: Velocity of sound does not depend upon the medium.
(a) both assertion and reason are true and the reason is the correct explanation of the assertion.
(b) both assertion and reason are true but reason is not the correct explanation of the assertion.
(c) Assertion is true but reason is false.
(d) Assertion is false but the reason is true.
Answer:
(c) The assertion is true but the reason is false.
Explanation: Velocity of sound increases with increase in humidity. The velocity of sound depends upon medium.
V. Answer Very Briefly
Question 1.
What is sound? Is it a force or energy?
Answer:
Sound is a vibration that typically propagates as an audible wave of pressure, through a transmission medium such as a gas, liquid or solid. Sound is not a force, it is an energy.
Question 2.
In which type of media, can longitudinal waves be propagated?
Answer:
Longitudinal waves can be propagated through solids, liquids and gases.
Question 3.
How does sound reach our ears from various sources?
Answer:
Sound reaches our ears in the form of transverse waves or vibrations from its source of production.

Question 4.
What is the minimum distance between two points in a wave having a phase difference of 2π?
Answer:
The minimum distance is the wavelength.
Question 5.
Suppose you and your friend are on the Moon. Will you be able to hear any sound produced by your friend?
Answer:
- As the Moon does not have air, you will not be able to hear any sound produced by your friend.
- Hence, you understand that the sound produced due to the vibration of different bodies needs a material medium like air, water, steel, etc, for its propagation.
- Hence, sound can propagate through a gaseous medium or a liquid medium or a solid medium.
Question 6.
What is the effect of pressure on the speed of sound in gas?
Answer:
The increase of pressure has no effect on the speed of sound in a gas.
Question 7.
Write down the difference between the sound and light waves.
Answer:
| Sound |
Light |
| 1. Medium is required for the propagation |
1. Medium is not required for the propagation. |
| 2. Sound waves are longitudinal. |
2. Light waves are transverse. |
| 3. Wavelength ranges from 1.65 cm to 1.65 m. |
3. Wavelength ranges from 4 × 10-7 m to 7 × 10-7 m. |
| 4. Sound waves travel in the air with a speed of about 340 ms-1 at NTP. |
4. Light waves travel in the air with a speed of 3 × 108 ms-1 |
Question 8.
Define particle velocity.
Answer:
The velocity with which the particles of the medium vibrate in order to transfer the energy in the form of a wave is called particle velocity.
Question 9.
Define wave velocity.
Answer:
The velocity with which the wave travels through the medium is called wave velocity.
Question 10.
Define velocity of a sound wave.
Answer:
The distance travelled by a sound wave in unit time is called the velocity of a sound wave.
\(\text { Velocity }=\frac{\text { Distance }}{\text { Time taken }}\)
Question 11.
State the laws of reflection of sound.
Answer:
The incident wave, the normal to the reflecting surface and the reflected wave at the point of incidence lie in the same plane.
The angle of incidence ∠i is equal to the angle of reflection ∠r.
Question 12.
State ‘Laws of reflection’.
Answer:
- The incident wave, the normal to the reflecting surface and the reflected wave at the point of incidence lie in the same plane.
- The angle of incidence ∠i is equal to the angle of reflection ∠r.
Question 13.
What is meant by rarer and denser medium?
Answer:
- Rarer medium: The medium in which the velocity of sound increases compared to other medium is called rarer medium. (Water is rarer compared to air for sound).
- Denser medium: The medium in which the velocity of sound decreases compared to other medium is called denser medium. (Air is denser compared to water for sound)
VI. Problem Corner
Question 1.
A wave of length 0.60 cm is produced in air and travels with a velocity of 340 ms-1. Will it be audible to the human ear?
Solution:
Wavelength, λ = 0.6 × 10-2 m
Velocity of sound, v = 340 ms-1
Frequency, n = \(\frac{v}{\lambda}\)
⇒ n = \(\frac{340}{0.6 \times 10^{-2}}\)
⇒ n = 566.66 × 102
⇒ n = 567 × 102 Hz.
Question 2.
Consider a source moving towards a listener at a speed of 0.9 v. Where v is the velocity of sound. Calculate the apparent frequency if the actual frequency is 600 Hz.
Solution:
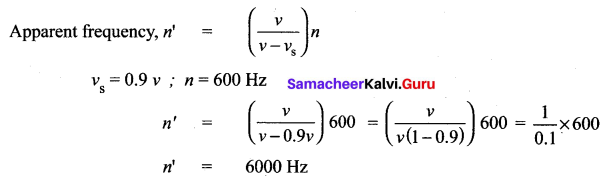
Question 3.
Audible frequencies have a range of 20 Hz to 20,000 Hz. Express it in terms of wavelength in air.
Answer:
Let the velocity of sound in air be 340 m/s
Initial frequency v1 = 20 Hz
Initial wavelength λ1 = \(\frac{v}{V_1}\)
= \(\frac{340}{20}\) = 17 m
Final frequency v2 = 20 × 10³ Hz
Final wavelength λ2 = \(\frac{340}{20×10³}\)
= 17 × 10-3 = 0.017 m
The range of wavelength is from 17 m to 0.017 m
VII. Answer in detail.
Question 1.
Write down the applications of echo?
Answer:
Applications of echo:
- Some animals communicate with each other over long distances and also locate objects by sending the sound signals and receiving the echo as reflected from the targets.
- The principle of echo is used in obstetric ultrasonography, which is used to create real – time visual images of the developing embryo or fetus in the mother’s uterus. This is a safe testing tool, as it does not use any harmful radiations.
- Echo is used to determine the velocity of sound waves in any medium.
Question 2.
Classify sound waves based on their frequencies.
Answer:
(i) Audible waves : These are sound waves with a frequency ranging between 20 Hz and 20,000 Hz. These are generated by vibrating bodies such as vocal cords, stretched strings etc.
(ii) Infrasonic waves : These are sound waves with a frequency below 20 Hz that cannot be heard by the human ear. eg: waves produced during earth quake, ocean waves, sound produced by whales, etc.
(iii) Ultrasonic waves : These are sound waves with a frequency greater than 20 kHz, Human ear cannot detect these waves, but certain creatures like mosquito, dogs, bats, dolphins can detect these waves, eg: waves produced by bats.
Question 3.
Write down the concept of Ear trumpet and Megaphone.
Answer:
Ear trumpet:
- Ear trumpet is a hearing aid, which is useful by people who have difficulty in hearing.
- In this device, one end is wide and the other end is narrow. The sound from the sources fall into the wide end and are reflected by its walls into the narrow part of the device. This helps in concentrating the sound and the sound enters the eardrum with more intensity. This enables a person to hear the sound better.
Megaphone:
- A megaphone is a horn – shaped device used to address a small gathering of people.
- It’s one end is wide and the other end is narrow. When a person speaks at the narrow end, the sound of his speech is concentrated by the multiple reflections from the walls of the tube. Thus, his voice can be heard loudly over a long distance.
Question 4.
What is the Doppler effect? Derive the formula for the change in apparent frequency
1. Both source and listener move towards and away from each other.
2. Both source and listener move one behind the other source follows the listener and listener follows the source.
3. A source at rest, listener moves towards and away from the source.
4. A listener at rest, the source moves towards and away from the listener.
Answer:
1.
| Position of source and listener |
Note |
Expression for apparent frequency |
(i) Both source and listener move
(ii) They move towards each other |
(a) Distance between source and listener decreases.
(b) Apparent frequency is more than actual frequency. |
\(n^{\prime}=\left(\frac{v+v_{\mathrm{L}}}{v-v_{\mathrm{S}}}\right) n\) |
(i) Both source and listener move
(ii) They move away from each other |
(a) Distance between source and listener increases.
(b) Apparent frequency is less than actual frequency.
(c) vS and vL become opposite to that in case-1. |
\(n^{\prime}=\left(\frac{v-v_{\mathrm{L}}}{v+v_{\mathrm{S}}}\right) n\) |
2.
| Position of source and listener |
Note |
Expression for apparent frequency |
(i) Both source and listener move
(ii) They move one behind the other
(iii) Source follows the listener |
(a) Apparent frequency depends on the velocities of the source and the listener.
(b) vS becomes opposite to that in case-2. |
\(n^{\prime}=\left(\frac{v-v_{\mathrm{L}}}{v-v_{\mathrm{S}}}\right) n\) |
(i) Both source and listener move
(ii) They move one behind the other
(iii) The listener follows the source |
(a) Apparent frequency depends on the velocities of the source and the listener.
(b) vS and vL become opposite to that in case-3. |
\(n^{\prime}=\left(\frac{v+v_{\mathrm{L}}}{v+v_{\mathrm{s}}}\right) n\) |
3.
| Position of source and listener |
Note |
Expression for apparent frequency |
(i) Source at rest
(ii) Listener moves towards the source |
(a) Distance between source and listener decreases.
(b) Apparent frequency is more than actual frequency.
(c) vS = 0 in case-1. |
\(n^{\prime}=\left(\frac{v+v_{\mathrm{L}}}{v}\right) n\) |
(i) Source at rest
(ii) Listener moves away from the source |
(a) Distance between source and listener increases.
(b) Apparent frequency is less than actual frequency.
(c) vS = 0 in case-2. |
\(n^{\prime}=\left(\frac{v-v_{\mathrm{L}}}{v}\right) n\) |
4.
| Position of source and listener |
Note |
Expression for apparent frequency |
(i) Listener at rest
(ii) Source moves towards the listener |
(a) Distance between source and listener decreases.
(b) Apparent frequency is more than actual frequency.
(c) vL = 0 in case-1. |
\(n^{\prime}=\left(\frac{v}{v-v_{\mathrm{S}}}\right) n\) |
(i) Listener at rest
(ii) Source moves away from the listener |
(a) Distance between source and listener increases.
(b) Apparent frequency is less than actual frequency.
(c) vL = 0 in case-2. |
\(n^{\prime}=\left(\frac{v}{v+v_{\mathrm{S}}}\right) n\) |
Question 5.
Explain echo method to measure velocity’ of sound.
Answer:
Apparatus required: A source of sound pulses, a measuring tape, a sound receiver, and a stop watch.
Procedure:
- Measure the distance ‘d’ between the source of sound pulse and the reflecting surface using the measuring tape.
- The receiver is also placed adjacent to the source. A sound pulse is emitted by the source.
- The stopwatch is used to note the time interval between the instant at which the sound pulse is sent and the instant at which the echo is received by the receiver. Note the time interval as ‘t’.
- Repeat the experiment for three or four times. The average time taken for the given number of pulses is calculated.
Calculation of speed of sound : The sound pulse emitted by the source travels a total distance of 2d while travelling from the source to the wall and then back to the receiver. The time taken for this has been observed to be ‘t’. Hence, the speed of sound wave is given by:
Speed of sound = \(\frac{distence Travelled}{Time Taken}\)
= \(\frac{2d}{t}\)
![]()
![]()

![]()
![]()

![]()