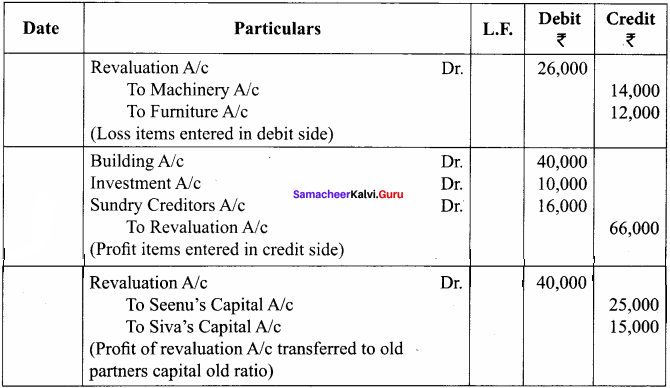
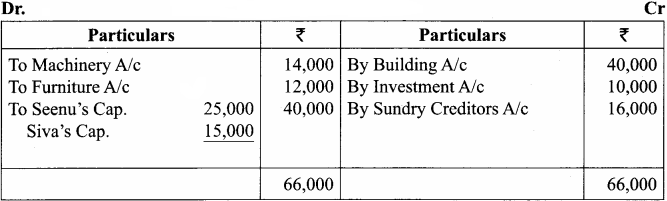
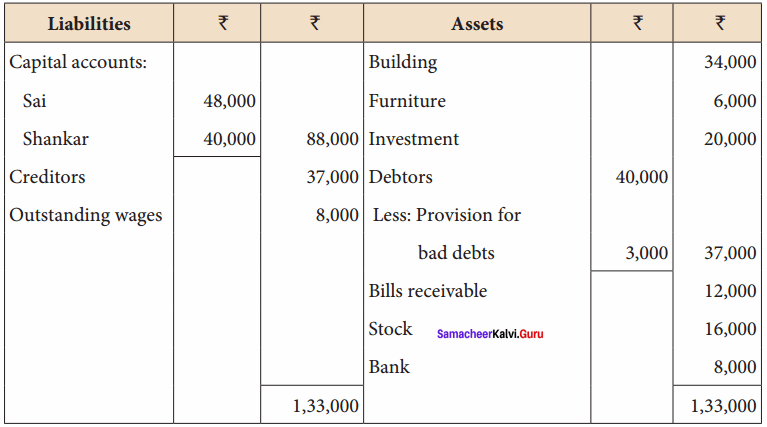
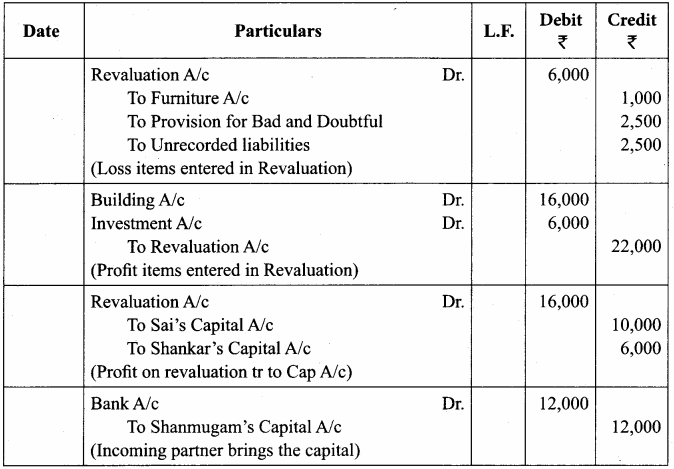
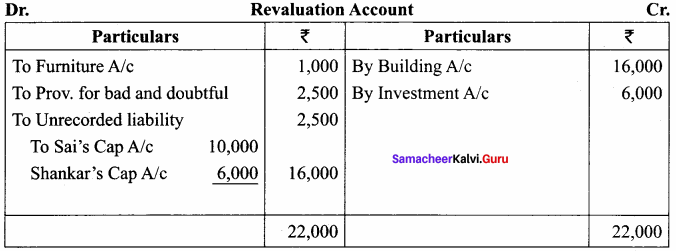
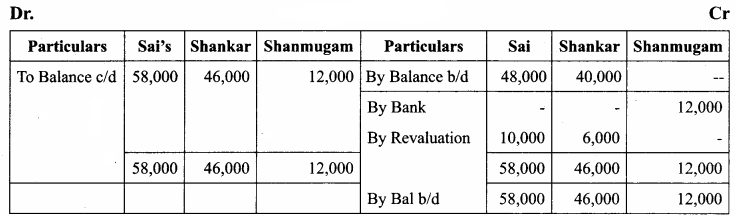
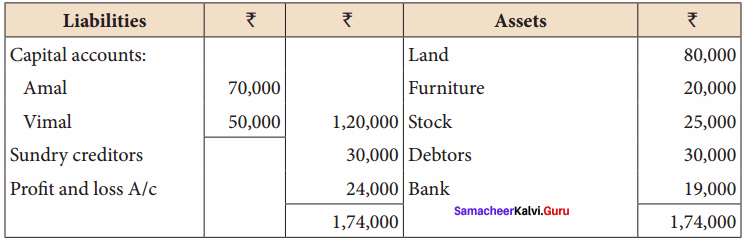
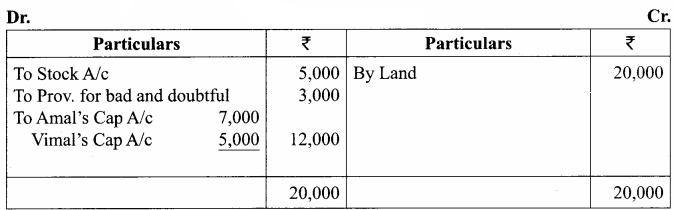
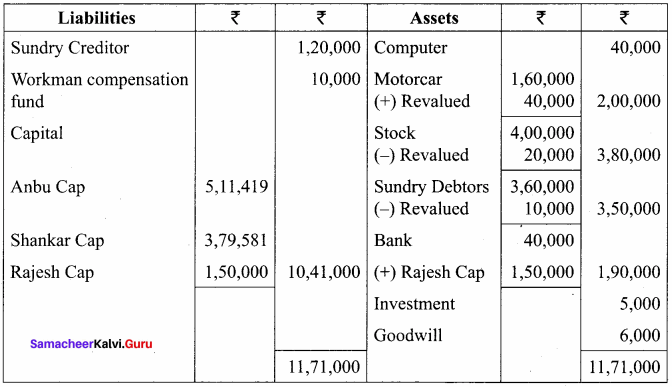
Students can Download Chemistry Chapter 10 Chemical Bonding Questions and Answers, Notes Pdf, Samacheer Kalvi 11th Chemistry Solutions Guied Pdf helps you to revise the complete Tamilnadu State Board New Syllabus and score more marks in your examinations.
Tamilnadu Samacheer Kalvi 11th Chemistry Solutions Chapter 10 Chemical Bonding
Samacheer Kalvi 11th Chemistry Chapter 10 Chemical Bonding Textual Evaluation Solved
Samacheer Kalvi 11th Chemistry Chemical Bonding Multiple Choice Questions
Question 1.
In which of the following compounds does the central atom obey the octet rule?
(a) XeF4
(b) AICI3
(c) SF6
(d) SO2
Answer:
(d) SCl2
Solution:

Hence in (d) SCl2 octet rule is followed
Question 2.
In the molecule OA = C = OB the formal charge on OA, C and OB are respectively.
(a) – 1, 0, +1
(b) +1, 0, – 1
(c) – 2, 0, +2
(d) 0, 0, 0
Answer:
(d) 0, 0, 0
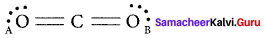
Formal charge of 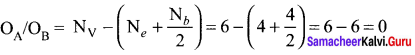
Formal charge of C = 4 – \(\left( 0+\frac { 8 }{ 2 } \right)\) = 4 – 4 = 0
Question 3.
Which of the following is electron deficient?
(a) PH3
(b) (CH3)2
(c) BH3
(d) NH3
Answer:
(c) BH3
Solution:
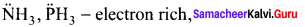 – electron rich,
– electron rich,
CH3 – CH3 – Covalent neutral molecule,
BH3 – electron deficient
Question 4.
Which of the following molecule contain no π bond?
(a) SO2
(b) NO2
(c) CO2
(d) H2O
Answer:
(d) H2O
Solution:
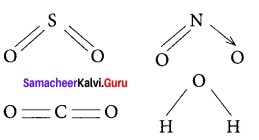
Water (H2O) contains only σ bonds and no π bonds.
Question 5.
The ratio of number of sigma (σ) and pi (π) bonds in 2- butynal is …………..
(a) 8/3
(b) 5/3
(c) 8/2
(d) 9/2
Answer:
(d) 9/2
Solution:
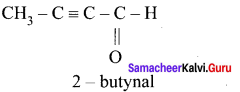
no. of σ bonds = 8 [4C – H; 3C – C; 1C— O]
no.of π bonds = 3 [2C – C; 1C – O]
∴ratio = \(\frac { 8 }{ 3 }\)
Question 6.
Which one of the following is the likely bond angles of sulphur tetrafluoride molecule?
(a) 120°, 80°
(b) 109°, 28°
(c) 90°
(d) 89°, 117°
Answer:
(d) 89°, 117°
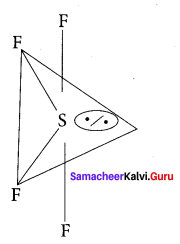
Solution:
Normal bond angle in regular trigonal bipyramidal are 90° and 120°. Due to l.p – b.p repulsion, bond angle is reduced to 89°, 117° option (d).
Question 7.
Assertion: Oxygen molecule is paramagnetic.
Reason: It has two unpaired electron in its bonding molecular orbital.
(a) both assertion and reason are true and reason is the correct explanation of assertion
(b) both assertion and reason are true but reason is not the correct explanation of assertion
(c) assertion is true but reason is false
(d) Both assertion and reason are false
Answer:
(c) assertion is true but reason is false
Correct statement: Oxygen molecule is paramagnetic
Correct Reason: It has two unpaired electrons in its antibonding molecular orbital.
Question 8.
According to Valence bond theory, a bond between two atoms is formed when ……………….
(a) fully filled atomic orbitals overlap
(b) half filled atomic orbitals overlap
(c) non-bonding atomic orbitals overlap
(d) empty atomic orbitals overlap
Answer:
(b) half filled atomic orbitals overlap

Question 9.
In CIF3, NF3 and BF3 molecules the chlorine, nitrogen and boron atoms are …………………….
(a) sp3 hybridised
(b) sp3, sp3 and sp2 respectively
(c) sp2 hybridised
(d) sp3d, sp3 and sp2 hybridised respectively
Answer:
(d) sp3d, sp3 and sp2 hybridised respectively
Solution:
CIF3 – sp3d hybridisation
NF3 – sp3 hybridisation
BF3 – sp2 hybridisation
Question 10.
When one s and three p orbitais hybridise,
(a) four equivalent orbitais at 900 to each other will be formed
(b) four equivalent orbitais at 1090 28’ to each other will be formed.
(c) four equivalent orbitals, that are lying the same plane will be formed
(d) none of these
Answer:
(b) four equivalent orbitals at 109° 28′ to each other will be formed.

Question 11.
Which of these represents the correct order of their increasing bond order?
(a) C2 < C22- < O2
(b) C22- < C2+ < O2 < O22-
(c) O22- < O2+ < O2 < C22-
(d) O22- < C2+ < O2 < C22-
Answer:
(d) O22- < C2+ < O2 < C22-
Solution:
bond order = (nb – na)
bond order of O22- = \(\frac { 1 }{ 2 }\) (8 – 6) = 1
bond order of C2+ = \(\frac { 1 }{ 2 }\) (5 – 2) = 1.5
bond order of O2 = \(\frac { 1 }{ 2 }\) (8 – 4) = 2
bond order of C22- = \(\frac { 1 }{ 2 }\) (8 – 2) = 3
Question 12.
Hybridisation of the central atom in PCl5 involves the mixing of orbitais.
(a) s, px, py, dx2, dx2-y2
(b) s, px, py, pxy, dx2-y2
(c) s, px, py, pz, dx2-y2
(d) px, py, pxy, dx2-y2
Answer:
(c) s, px, py, pz, dx2-y2
Solution:
PCl5 – sp3d hybridisation s, px, py, pz, dx2-y2
Question 13.
The correct order of O – O bond length in hydrogen peroxide, ozone, and oxygen are……………..
(a) H2O2 > O3 > O2
(b) O2 > O3 > H2O
(c) O2 > H2O2 > O3
(d) O3 > O2 > H2O2
Answer:
(b) O2 > O3 > H2O2
Solution:
The bond order for O2, O3 and H2O2 decreases in the order 2 > 1.5 > 1
Question 14.
Which one of the following is diamagnetic?
(a) O2
(b) O22-
(c) O22+
(d) None of these
Answer:
(b) O22-
Solution:
O22- is diamagnetic. Additional two electrons are paired in anti-bonding molecular orbits π*2py and π*2pz
Question 15.
Bond order of a species is 2.5 and the number of electrons is in its bonding molecular orbital is found to be 8. The no. of electrons in its anti-bonding molecular orbital is ………………….
(a) three
(b) four
(c) zero
(d) cannot be calculated from the given information.
Answer:
(a) three
Solution:
Bond order = \(\frac { 1 }{ 2 }\) (nb – na)
2.5 = \(\frac { 1 }{ 2 }\) (8 – na)
⇒ 5 = 8
⇒ na = 8 – 5 = 3

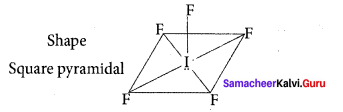
Question 16.
Shape and hybridisation of IF5 are ………….
(a) Trigonal bipyramidal, sp3d2
(b) Trigonal bipyramidal, sp3d
(c) Squai2e pyramidal, sp3d2
(d) Octahedral, sp3d2
Answer:
(c) Square pyramidal, sp3d2
Solution:
IF5 – 5 bond pair + 1 lone pair
∴ hybridisation sp3d2
Question 17.
Pick out the incorrect statement from the following.
(a) sp3 hybrid orbitais are equivalent and are at an angle of 1 09°28’ with each other.
(b) dsp2 hybrid orbitais are equivalent and bond angle between any two of them is 900.
(c) All five sp3d hybrid orbitais arc not equivalent. Out of these five sp3d hybrid orbitais, three are at an angle of 120°, remaining two are perpendicular to the plane containing the other three
(d) none of these
Answer:
(c) All five sp3d hybrid orbitals are not equivalent. Out of these five sp3d hybrid orbitals, three are at an angle of 120° remaining two are perpendicular to the plane containing the other three.
Question 18.
The molecules having same hybridisation, shape and number of lone pairs of electrons are ………………..
(a) SeF4, XeO2F2
(b) SF4, XeF2
(c) XeOF4, TeF4
(d) SeCI4, XeF4
Answer:
(a) SeF4, XeO2F2
Solution:
SeF4, XeO2F2 – sp3d hybridisation
T – shaped, one lone pair on central atom.
Question 19.
In which of the following molecules / ions BF3, NO2–, H2O the centrai atom is sp2 hybridised?
(a) NO2– and H2O
(b) NO2– and H2O
(c) BF3 and NO2
(d) BF3 and NH2–
Answer:
(c) BF3 and NO2
Solution:
H2O – Central atom sp3 hybridised
NO2– – Central atom sp2 hybridised
BF3 – Central atom sp2 hybridised
NH2– – Central atom sp3 hybridised

Question 20.
Some of the following properties of two species, NO3– and H3O+ are described below. Which one of them is correct?
(a) dissimilar in hybridisation for the central atom with different structure.
(b) isostnictural with same hybridisation for the Central atom.
(c) different hybridisation for the central atom with same structure
(d) none of these
Answer:
(a) dissimilar in hybridisation for the central atom with different structure.
Solution:
NO3– – sp2 hybridisation, planar
H3O+ – sp3 hybridisation, pyramidal
Question 21.
The types of hybridisation on the five carbon atom from right to left in the, 2,3 pentadiene.
(a) sp3, sp2, sp, sp2, sp3
(b) sp3, sp, sp, sp, sp3
(c) sp2, sp, sp2, sp , sp3
(d) sp3, sp3, sp2, sp3, sp3
Answer:
(a) sp3, sp2, sp, sp2, sp3
Solution:
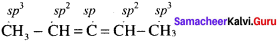
Question 22.
XeF2 is isostructural with ………..
(a) SbCl2
(b) BaCl2
(c) TeF2
(d) ICl2–
Answer:
(d) ICl2–
Solution:
XeF2 is isostructural with ICI2–
Question 23.
The percentage of s-character of the hybrid orbitais in methane, ethane, ethene and ethyne are respectively ………………..
(a) 25, 25, 33.3, 50
(b) 50, 50, 33.3, 25
(c) 50, 25, 33.3, 50
(d) 50, 25, 25. 50
Answer:
(a) 25, 25, 33.3, 50
Solution:
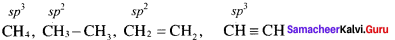
Question 24.
Of the following molecules, which have shape similar to carbon dioxide?
(a) SnCI2
(b) NO2
(c) C2H2
(d) All of these
Answer:
(c) C2H2
Solution:
CO2 – Linear
C2H2 – Linear
Question 25.
According to VSEPR theory, the repulsion between different parts of electrons obey the order …………………
(a) l.p – l.p > b.p – b.p > l.p – b.p
(b) b.p – b.p > b.p – 1.p > l.p – b.p
(c) l.p – l.p > b.p – l.p > b.p – b.p
(d) b.p – b.p > l.p – l.p > b.p – l.p
Answer:
(c) l.p – l.p > b.p – l.p > b.p – b.p

Question 26.
Shape of CIF3 is ……………………..
(a) Planar triangular
(b) Pyramidal
(c) ‘T’ Shaped
(d) none of these
Answer:
(c) ‘T’ Shaped
Solution:
dF3 – sp3d hybridisation
Question 27.
Non- Zero dipole moment is shown by …………………
(a) CO2
(b) p – dichlorobenzene
(c) carbon tetrachloride
(d) water
Answer:
(d) water
Solution:
Question 28.
Which of the following conditions is not correct for resonating structures?
(a) the contributing structure must have the same number of unpaired electrons.
(b) the contributing structures should have similar energies.
(c) the resonance hybrid should have higher energy than any of the contributing structure.
(d) none of these
Answer:
(c) the resonance hybrid should have higher energy than any of the contributing structure.
Solution:
Correct statement is – the resonance hybrid should have lower energy than any of the contributing structure.
Question 29.
Among the following, the compound that contains, ionic, covalent and coordinate linkage is …………………
(a) NH4Cl
(b) NH3
(c) NaCl
(d) none of these
Answer:
(a) NH4Cl
Question 30.
CaO and NaCl have the same crystal structure and approximately the same radii. If U is the lattice energy of NaCl, the approximate lattice energy of CaO is ………….
(a) U
(b) 2U
(c) U/2
(d) 4U
Answer:
(d) 4U
Samacheer Kalvi 11th Chemistry Chemical Bonding Short Answer Questions.
Question 31.
Define the following
- Bond order
- Hybridisation
- a- bond
Answer:
1. Bond order:
Bond order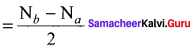
The number of bonds formed between the two bonded atoms in a molecule is called bond order.
2. Hybridisation:
It is a process of mixing of atomic orbitais of the same atom with. comparable energy to form equal number of new equivalent orbitais with same energy. The resultant orbitais are called hybridised orbitais and they possess maximum symmetry and definite orientation in space so as to minimise the force of repulsion between their electrons.
3. σ – bond:
When two atomic orbitais overlap linearly along the axis, the resultant bond is called sigma (σ) bond.
Question 32.
What is a pi bond?
Answer:
Pi – bond:
When two atomic orbitals overlap sideways, the resultant covalent bond is called a pi (π) bond.

Question 33.
In CH4, NH3 and H2O, the central atom undergoes sp3 hybridisation-yet their bond angles are different, why?
Answer:
1. In CH4, NH3 and H2O the central atom undergoes sp3 hybridisation. But their bond angles are different due to the presence of lone pair of electrons.
2. It can be explained by VSEPR theory. According to this theory, even though the hybridisation is same, the repulsive force between the bond pairs and lone pairs arc not same.
3. Bond pair-Bond pair < Bond pair – Lone pair < Lone pair – Lone pair So due to the varying repulsive force the bond pairs and lone pairs are distorted from regular geometry and organise themselves in such a way that repulsion will be minimum and stability will be maximum.
4. In case of CH4, there are 4 bond pairs and no lone pair of electrons. So it remains in its regular geometry. i.e., tetrahedral with bond angle 109° 28’.
5. H2O has 2 bond pairs and 2 lone pairs. There is large repulsion between lp – lp. Again repulsion between lp – bp is more than that of 2 bond pairs. So 2 bonds are more restricted to form inverted V shape (or) bent shape molecule with a bond angle of 104° 35’.
6. NH3 has 3 bond pairs and 1 lone pair. There is repulsion between lp – bp. So 3 bonds are more restricted to form pyramidal shape with bond angle equal to 107° 18’.
Question 34.
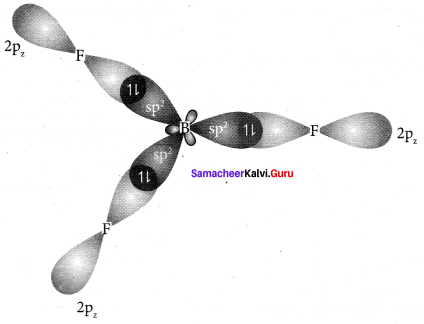
Explain sp2 hybridisation in BF3
Answer:
1. sp2 hybridisation in boron trifluoride – Boron atom – B. Electronic configuration [H2]2s22p2.
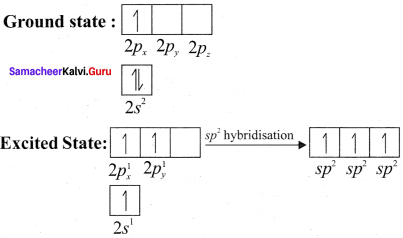
2. In boron, the s orbital and two p orbitals in the valence shell hybridises to generate three equivalent sp2 orbitais. These 3 orbitaIs lie in the same xy plane and the angle between any two orbitals is equal to 120°.
3. The 3 sp2 hybridised orbitais of boron now overlap with the 2pz orbitais of fluorine (3 atoms). This overlap takes place along the axis.
Question 35.
Draw the M.O diagram for oxygen molecule and calculate its bond order and show that O2 is paramagnetic.
Answer:
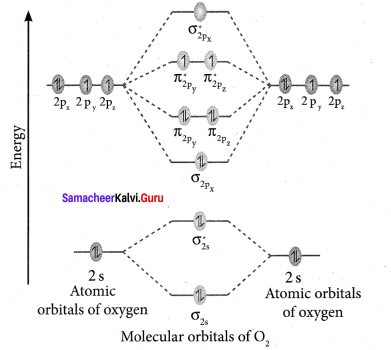
- Electronic configuration of O atom is is 1s2 2s2 2P4
- Electronic configuration of O, molecule is
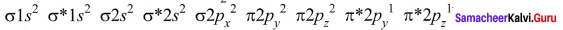
- Bond order =
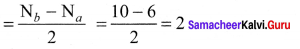
- Molecule has two unpaired electrons, hence it is paramagnetic.
Question 36.
Draw MO diagram of CO and calculate its bond order.
Answer:
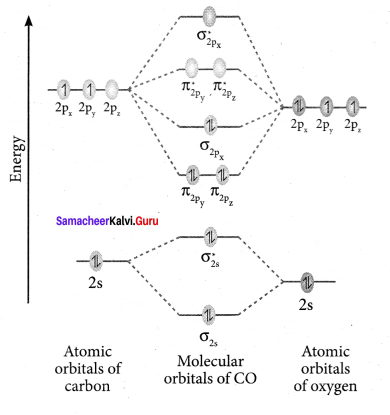
- Electronic configuration of C atom: 1s2 2s2 2p2
Electronic configuration of O atom: 1s2 2s2 2p4
- Electronic configuration of CO molecule is: σ1s2 σ*1s2 σ2s2 σ*22 π2py2 π2pz2 σ2px2
- Bond order
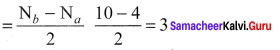
- Molecule has no unpaired electron, hence it is diamagnetic.
Question 37.
What do you understand by Linear combination of atomic orbitais in MO theory?
Answer:
Linear combination of atomic orbitais (LCAO):
1. The wave functions for the molecular orbitais can be obtained by solving the Schrodinger wave equation for the molecule. Since solving Schrodinger wave equation is too complex, a most common method linear combination of atomic orbitais (LCAO) is used to obtain wave function for molecular orbitals.
2. Atomic orbitais are represented by wave functions ψ. Consider two atomic orbitals represented by the wave functions ψA and ψB with comparable energy that combines to form two molecular orbitals.
3. One is bonding molecular orbitai (ψ bonding) and the other is anti-bonding molecular orbital (ψ anti-bonding).
4. The wave function for molecular orbitais, ψA and ψB can be obtained by the LCAO as shown below:
ψbonding = ψA + ψB
ψanti-bonding = ψA – ψB
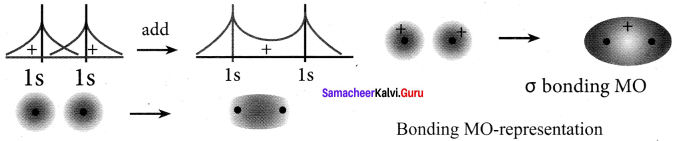
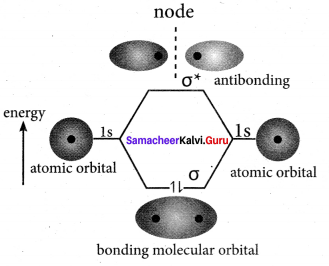
5. The formation of bonding molecular orbital can be considered as the result of constructive interference of the atomic orbitais and the formation of anti-bonding molecular orbital can be the result of the destructive interference of the atomic orbitais.
6. The formation of two molecular orbitals from two is orbitals is show below.
Constructive interaction:
The two is orbitals are in phase and have the same signs.
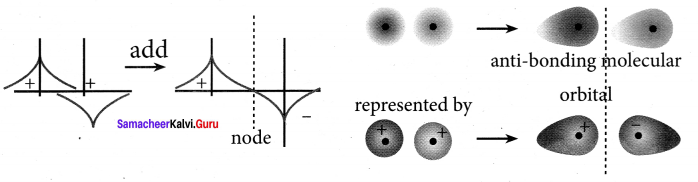
Destructive interaction:
The two is orbitals are out of phase and have opposite signs
Question 38.
Discuss the formation of N2 molecule using MO Theory.
Answer:
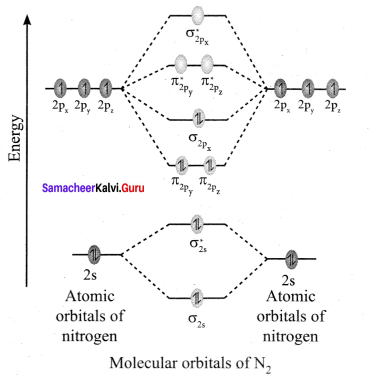
- Electronic configuration of N atom 1s2 2s2 2p3.
- Electronic configuration of N, molecule is: σ1s2 σ*1s2 σ2s2 σ*22 π2py2 π2pz2 σ2px2
- Bond order
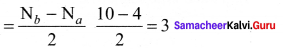
- Molecule has no unpaired electrons hence, it is diamagnetic.
Question 39.
What is dipole moment?
Answer:
1. The polarity of a covalent bond can be measured in terms of dipole moment which is defined as: µ = q x 2d, where µ is the dipole moment, q is the charge, 2d is the distance between the two charges.
2. The dipole moment is a vector quantity and the direction of the dipole moment points from the negative charge to positive charge.
3. The unit of dipole moment is Coulomb metre (C m). It is usually expressed in Debye unit (D).
4. 1 Debye = 3.336 x 10-30 Cm

Question 40.
Linear form of carbon dioxide molecule has two polar bonds. yet the molecule has zero dipole moment, why?
Answer:
- The linear form of carbon dioxide has zero dipole moment, even though it has two polar bonds.
- In CO2, there are two polar bonds [C = O], which have dipole moments that are equal in magnitude but have opposite direction.
- Hence the net dipole moment of the CO2 is µ = µ1 + µ2 = µ1 +( – µ1) = 0

- In this case
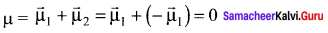
Question 41.
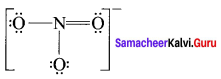
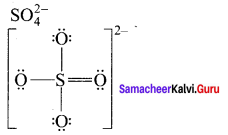
Draw the Lewis structures for the following species.
- NO3–
- SO42-
- HNO3
- O3
Answer:
1. NO3–
2. SO42-
3. HNO3
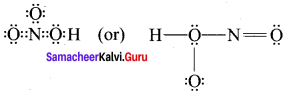
4. O3

Question 42.
Explain the bond formation in BeCl2 and MgCl2. BeCl2 bond formation:
Answer:
1. Electronic confiuration of Be(Z = 4) is 1s2 2s2 and electronic configuration of Cl (Z = 17) is 1s2 2s2 2p6 3s2 3p5.
2. Beryllium has 2 electrons in its valence shell and chlorine atoms (2) have 7 electrons in their valence shell.
3. By losing two electrons, Beryllium attains the inert gas configuration of Helium and becomes a dipositive cation, Be2+ and each chlorine atom accepts one electron to become (Cl–) uninegative anion and attains the stable electronic configuration of Argon.
4. Then Be2+ combine with 2Cl– ions to form an ionic crystal in which they are held together by electrostatic attractive forces.
5. During the formation of 1 mole of BeCl2, the amount of energy released is – 468 kJ/mol. This favours the formation of BeCl, and its stabilisation.
MgCI2 bond formation:
1. Electronic configuration of Mg (z = 12) is 1s2 2s2 2p6 3s2.
Electronic configuration of Cl (z = 17) is 1s2 2p6 3p6 3p5
2. Magnesium has 2 electrons in its valence shell and chlorine has 7 electrons in its valence shell.
3. By losing two electrons, magnesium attains the inert gas configuration of Neon and becomes a dipositive cation (Mg2+) and two chlorine atoms accept these electrons to become two uninegative anions [2Cl–] by attaining the stable inert gas configuration of Argon.
4. These ions, Mg2+ and 2Cl– combine to form an ionic crystal in which they are held together by electrostatic attractive forces.
5. The energy released during the formation of 1 mole of MgCl2 is – 783 kJ/mole. This favours the formation of MgCI2 and its stabilisation.

Question 43.
Which bond is stronger or π? Why?
Answer:
σ bond is stronger than π bond. A sigma bond is formed by head-on overlapping of orbital is more effective. Hence it is a stronger bond. But pi bonds are formed by sidewise overlapping of orbitals. The sidewise overlapping of orbitals is less effective than head-on overlapping. Hence it is a weaker bond.
Question 44.
Define bond energy.
Answer:
Bond energy:
Bond energy (or) Bond enthalpy is defined as the minimum amount of energy required to break one mole of a bond in molecules in their gaseous state. The unit of bond energy is kJ mol-1
Question 45.
Hydrogen gas is diatomic whereas inert gases are monoatomic – explain on the basis of MO theory.
Answer:
1. Hydrogen gas is diatomic. According to MO theory. which is based on quantum mechanics H2 molecule can be represented in terms of the following diagram called M.O. diagram.
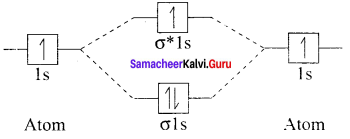
H – H. i.e.. H2 molecule has two atoms which are connected by 1 σ bond. So it is diatomic.
2. But in the case of inert gases. the valence shell is fully filled i.e.. an octet (8 electrons) (or) duplet (2 electrons) in case of Helium, due to which they are in monoatomic state and remain stable. So they do not combine with any atom (neither of same or of different elements). Due to this they do no exist in diatomic state and always exist in mono – atomic state.

Question 46.
What is Polar Covalent bond? Explain with example.
Answer:
1. If a covalent bond is formed between atoms having different electronegativities. the atom with higher electronegativity will have greater tendency to attract the shared pair of electrons towards itself than the other atom. As a result, the cloud of shared electron pair gets distorted and polar covalent bond is formed.
2. Example – HF – Hydrogen fluoride:
The electronegativities of hydrogen and fluorine on Pauling’s scale are 2.1 and 4 respectively. It means that fluorine attracts the shared pair of electrons approximately twice as much as hydrogen which leads to partial negative charge on the fluorine atom and partial positive charge on hydrogen atom. Hence, the H – F bond is said to be a polar covalent bond.
Question 47.
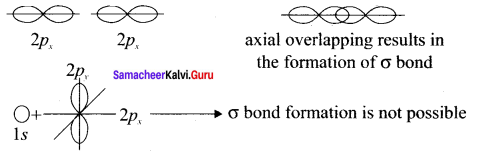
Considering x-axis as molecular axis, which out of the following will form a sigma bond.
- 1s and 2py
- 2px and 2px
- 2px and 2pz
- 1s and 2Pz
Answer:
Along X-axis as molecular axis, only 2p and 2p can form a sigma bond
Question 48
Explain resonance with reference to carbonate ion
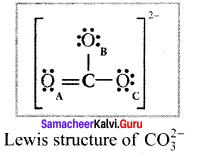
1. For the above structure, we can draw two additional lewis structures by moving the lone pairs from the other two oxygen atoms OB and OC. and thus creating three similar structures in which the relative positive of the atoms are same.
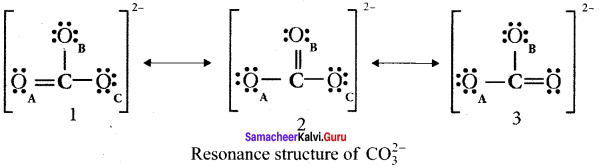
2. They only differ in the position of bonding and lone pair of electrons. Such structures are called resonance structures and this phenomenon is called resonance.
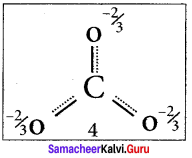
3. It is evident from the experimental results that all carbon-oxygen bonds in carbonate ion are equivalent. The actual structure of the molecule is said to be a resonance hybrid, an average of these 3 resonance forms. The following structure gives a qualitative idea about the correct structure of CO32- (carbonate) ion.
Question 49.
Explain the bond formation in ethylene and acetylene.
Answer:
Bonding in Ethylene, C2H4
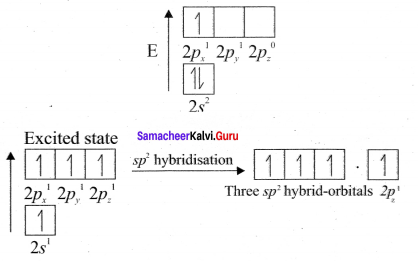
1. Bonding in ethylene can he explained by hybridisation concept.
2. The valency of carbon is 4. The electronic configuration of carbon is 1s2 2s2 2px1 2py1 2pz0. One electron from 2s orbital is promoted to 2pz. orbital in the excited state to satisfy the valency of carbon.
3. In ethylene both the carbon atoms undergo sp2 hybridisation involving 2s, 2px and spy orbitals resulting in 3 equivalent sp2 hybridised orbitals lying in the XY plane at an angle of 1200 to each other. The unhybridised 2pz orbital lies perpendicular to the xy plane.
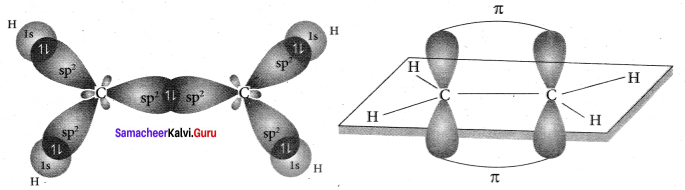
4. One of the sp2 hybndised orbitals of each carbon atoms lying along the X – axis linearly overlaps with each other resulting in the formation of C – C sigma bond. The other two sp2 hybridised orbitals of both carbon atom linearly overlap with the four is orbitals of four hydrogen atoms leading to the formation of two C – H sigma bonds on each carbon atom.
5. The unhybridised 2pz orbital of both carbon atoms can overlap only sideways as they are not in the molecular axis. This lateral overlap results in the formation of a pi bond between the two carbon atoms.
Bonding in acetylene (C2H2):
1. The electronic configuration of valence shell of carbon atom in the ground state is [He] 2s2 2px1 2pz0. One electron from 2s orbital is promoted to 2pz orbital in the excited state to satisfy the valency of carbon.
2. In acetylene molecule, both the carbon atoms are in sp hybridised state. The 2s and 2px orbitals resulting in two equivalent sp hybridised orbitals are formed lying in a straight line along the X – axis. The unhybridised 2py, and 2pz orbitais lie perpendicular to the X-axis.
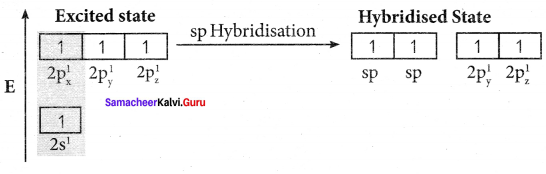

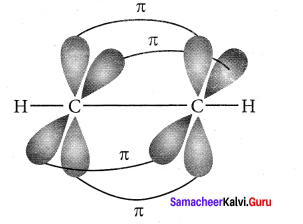
3. One of the two sp hybridised orbitals of each carbon atom linearly overlaps with each other resulting in the formation of a C – C sigma bond. The other sp hybridised orbital of both carbon atoms linearly overlap with the two is orbitals of two hydrogen atoms leading to the formation of one C – H sigma bond on each carbon atom.
4. The unhybridised 2py and 2pz orbitals of each carbon atom overlap sideways. This lateral overlap results in the formation of two pi bonds. (py – py) and (pz – pz) between the two carbon atoms.
Question 50.
What type of hybridisations are possible ¡n the following geometeries?
- octahedral
- tetrahedral
- square planar.
Answer:
- Octahedral geometry is possible by sp3d2 (or) d2sp3 hybridisation.
- Tetrahedral geometry is possible by sp3 hybridisation.
- Square planar geometry is possible by dsp2 hybridisation.

Question 51.
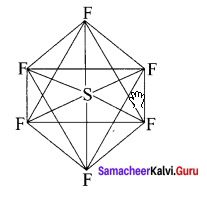
Explain VSEPR theory. Applying this theory to predict the shapes of F7 and SF6.
Answer:
VSEPR theory:
1. The shape of the molecules depend on the number of valence shell electron pair around the central atom.
2. There are two types of electron pairs namely, bond pairs and lone pairs.
3. Each pair of valence electrons around the central atom repel each other and hence they are located as far away as possible in three dimensional space to minimise the repulsion between them.
4. The repulsive interaction between the different types of electron pairs is in the following
order:
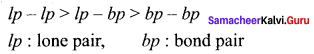
5. The lone pair of electrons are localised only on the central atom and interact with only one nucleus whereas the bond pairs are shared between two atoms and they interact with two nuclei. Because of this, the lone pairs occupy more space and have greater repulsive power than the bond pairs in a molecule.
IF7:
It is an AB7 type molecule. This molecule has 7 bond pair of electrons and no lone pair of electrons. Due to bond pair-bond pair interaction of electrons, IF7 has pentagonal bipyramidal shape.
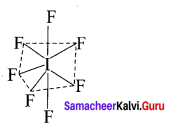
SF6:
It is an AB6 type molecule. This molecule has 6 bond pairs of electrons and no lone pair of electrons. Due to bond pair-bond pair interaction of electrons, SF6 has octahedral shape.
Question 52.
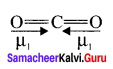
CO2 and H2O both are triatomic molecules but their dipole moment values are different. Why?
Answer:
1. Linear form of carbon dioxide has zero dipole moment. In CO2 the dipole moment of two polar bonds are equal in magiitude but have opposite direction. Hence, the net dipole moment of the CO2 molecule is
µ = µ1 + µ2
µ = µ1 + (- µ1) = 0
In this case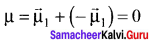
2. But in the case of water, net dipole moment is the vector sum µ1 + µ2 as follows:
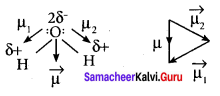
Dipole moment in water is found to be 1.85 D.
3. CO2 and H2O both are triatomic molecules but their dipole moment values are zero and 1.85 D respectively.
Question 53.
Which one of the following has highest bond order? N2, N2+ or N2– ?
Answer:
N2 (14 electrons)
Bond order = 3, 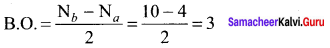
N2+ (13 electrons)
Bond order = 2.5, 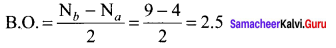
N2–(15 electrons)
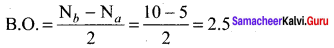
So N2 has the highest bond order.
Question 54.
Explain the covalent character in ionic bond.
Answer:
1. Ionic compounds like lithium chloride shows covalent character and it is soluble in organic solvents such as ethanol.
2. The partial covalent character in ionic compounds can be explained on the basis of a phenomenon called polarisation.
3. In an ionic compound, there is an electrostatic attractive force between the cation and anion. The positively charged cation attract the valence electrons of anion while repelling the nucleus.
This cause a distortion in the electron cloud of the anion and its electron density drills towards the cation, which results in some sharing of valence electrons between these ions. Thus, a partial covalent character is developed between them. This phenomenon is called polarisation.
4. Thus due to polarisation, ionic compounds shows covalent character.

Question 55.
Describe Fajan’s rule.
Answer:
(i) To show greater covalent character, both the cation and anion should have high charge on them. The higher the positive charge on the cation, greater will be the attraction on the electron cloud of the anion. Similarly higher the magnitude of negative charge on the anion, greater is its polarisability. Hence, the increase in charge on cation or in anion increases the covalent character.
Let us consider three ionic compounds aluminum chloride, magnesium chloride and sodium chloride. Since the charge of the cation increase in the order Na+ < Mg2+ < Al3+ the covalent character also follows the same order NaCl < MgCl2 < AlCl3.
(ii) The smaller cation and larger anion show greater covalent character due to the greater extent of polarisation. Lithium chloride is more covalent than sodium chloride. The size of Li+ is smaller than Na+ and hence the polarizing power of Li+ is more. Lithium iodide is more covalent than lithium chloride as the size of I– is larger than the Cl–. Hence I– will be more polarized than Cl– by the cation, Li+.
(iii) Cations having ns2 np6 nd10 configuration show greater polarizing power than the cations with ns2 np6 configuration. Hence, they show greater covalent character. CuCl is more covalent than NaCl. Compared to Na+ (1.13 Å). Cu+(0.6 Å) is small and have 3s2 3p6 3d10 configuration.
Electronic configuration of Cu+: [Ar] 3s2, 3p6, 3d10
Electronic Configuration of Na+: [He] 2s2, 2p6.
Samacheer Kalvi 11th Chemistry Chemical Bonding In Text Questions Evaluate yourself
Question 1.
Draw the lewis structures for
- Nitrous acid (HNO2)
- Phosphoric acid
- Sulphurtroxide (SO3)
Answer:
1. Nitrous acid (HNO2)

2. Phosphoric acid
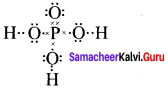
3. Sulphur troxide (SO3)
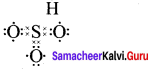
Question 2.
Calculate the formal charge on each atom of carbonyl chloride (COCl2)
Answer:
Formal charge 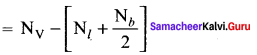
Carbonyl chloride COCl2
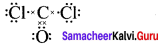
Formal charge on carbon atom = 4 – \(\left[ 0+\frac { 8 }{ 2 } \right]\) = 4 – 4 = 0
Formal charge on chlorine atom = 7 – \(\left[ 6+\frac { 2 }{ 2 } \right]\) = 7 – 7 = 0
Formal charge on oxygen atom = 6 – \(\left[ 4+\frac { 4 }{ 2 } \right]\) = 6 – 6 = 0
Question 3.
Explain the ionic bond formation in MgO and CaF2
Magnesium oxide (MgO):
Answer:
Electronic configuration of Mg – 1s2 2s2 2p5 3s2
Electronic configuration of O – 1s2 2s2 2p6 3s6 3p4.
1. Magnesium has two electrons in its valence shell and oxygen has six electrons in its valence shell.
2. By losing two electrons, Mg acquires the inert gas configuration of Neon and becomes a dipositive cation Mg2+
Mg → Mg2+ + 2e–
3. Oxygen accepts the two electrons to become a di negative oxide anion, O2- thereby attaining the inert gas configuration of Neon.
O + 2e– → O2-
4. These two ions, Mg2+ and O2- combine to form an ionic crystal in which they are held together by electrostatic attractive forces.
5. During the formation of magnesium oxide crystal 601.6 kJ mol-1 energy is released . This favours the formation of magnesium oxide (MgO) and its stabilisation.
CaF2, Calcium fluoride
1. Calcium, Ca: [Ar] 4s2, Fluorine F: [He] 2s2 2p5
2. Calcium has two electrons in its valence shell and fluorine has seven electrons in its valence shell.
3. By losing two electrons, calcium attains the inert gas configuration of Argon and becomes a dipositive cation, Ca2+.
4. Two fluorine atoms, each one accepts one electron to become two unincgative fluoride ions (F–) thereby attaining the stable configuration of Neon.
5. These three ions combine to form an ionic crystal in which they are held together by electrostatic attractive force.
6. During the formation of calcium fluoride crystal 1225.91 kJ mol-1 of energy is released. This favours the formation of calcium fluoride, CaF2 and its stabilisation.

Question 4.
Write the resonance structures for
- Ozone molecule
- N2O
Answer:
1. Ozone molecule, O3

2. Nitrous oxide, N2O
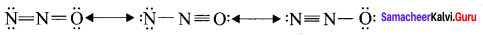
Question 5.
Of the two molecules OCS and CS2 which one has higher dipole moment value? Why?
Answer:
OCS and CS2
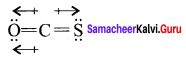
The dipole moment µOCS = 0.7 149 ± 0.0003 Debye.
CS2:
S = C = S
In CS2, the bond dipoles of 2C = S have same values and bond dipoles cancel each other so dipole moment of CS2 is zero. Among OCS and CS2, OCS has a higher dipole moment because in OCS oxygen is more electronegative than sulphur and C = S and C = O bonds in OCS molecules do not cancel each other.
On the other hand CS2, due to linear structure, the bond dipole of two C = S bonds cancel each other. On the other hand, CS2 due to linear structure, the bond dipoles of two C = S bonds cancel each other and thc recultant dipo’c moment value is zero. So OCS has a higher dipole moment than CS2.
Question 6.
Arrange the following in the decreasing order of Bond angle
- CH4, H2O, NH3
- C2H2, BF3, CCl4
Answer:
1. CH4, H2O, NH3:
NH3 = 107°, Water= 104.5°, CH4 = 109.5°
Decreasing order of bond angle: H2O < NH3 < CH4
2. C2H2, BF3, CCl4:
C2H2 = 1800, BF3 = 120°, CCl4 = 109.5°
Decreasing order of bond angle: CCl4 < BF3 < C2H2
Question 7.
Bond angle in PH4+ is higher than in PH3. Why?
Answer:
Phosphorous in both PH3 and PH4+ is sp3 hybridised. Due to the absence of lone pair-bond pair repulsion and presence of four identical bond pair-bond pair interactions, PH4+ assumes tetrahedral geometry with a bond angle of 109° 28’.
But PH3 has three bond pairs and one lone pair around P. Due to greater lone pair-bond pair repulsion than bond pair-bond pair repulsion, the tetrahedral angle decreases from 109° 28’ to 93.6°. As a result PH3 is pyramidal.
PH3 – Pyramidal with bond angle of 93.6°. PH4+ Tetrahedral with bond angle of 109° 28’.

Question 8.
Explain the bond formation in SF4 and CCl4 using hybridisation concept.
Answer:
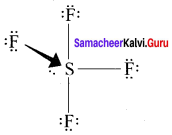
In SF4, the central atom is sp3d hybridised.
1. The molecule SF3 will have a total 34 valence electron 6 form sulphur, 7 each from four fluorine atoms.
2. Sulphur atom will from 4 single bonds with fluorine atoms. These bonds account for the 8 electrons out of the 34 valence electrons. Each fluorine atom will have 3 lone pair of electrons in order to have a complete octet structure.
These lone pairs will use up 24 valence electrons. So the total used valence electrons, are 32. The remaining 2 electrons will be placed on the sulphur atom as a lone pair. Sulphur atom gets a total of 10 electrons 8 from the bonds and 2 as lone pair.
3. This is quite Sulphur atom gets a total of 10 electrons 8 from the bonds and 2 as lone pair. This is quite possible for sulphur because it has easy access to its 3d orbital which means that it can expand its octet and accommodate more than 8 electrons.
4. Sulphur forms 4 single bonds and has 1 lone pair which means that its steric number is equal to 5. In this case sulphur will use five hybrid orbitais, such as one 3s orbital three 3p orbitais and one 3d orbital. So the central atom is sp3d hybridised.
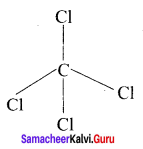
CCl4:
1. It is not necessary to invoke hybridisation especially in CCl4. It must be invoked for all tetrahedral bonds of carbon and other atoms.
2. The electronic configuration of an isolated carbon atom in its ground state is 1s2 s2 2p2.
3. CCl4 is a tetrahedral molecule comprising of four single bonds known as a bonds between the carbon atom and the chlorine atoms. In this type of bonding, the 2s orbital and three 2p orbitals of carbon atoms are mixed to produce four identical orbitals, a process known as sp3 hybridisation.
Question 9.
The observed bond length of N2+ is larger than N2 while the bond length in NO+ is less than in NO. Why?
Answer:
(a)
(1) By molecular orbital theory, the bond order of both N2+ is 2.5 whereas N2 is 3.
2. N2 has 5e– in the antibonding molecular orbital whereas N2+ has 4e– in the antibonding molecular orbital. So N2+ will make a stronger and shorter bond length.
3. More the bond order and bond strength, and lesser will be the bond length.
4. So we can easily conclude N2 has more bond length than N2
Bond order in 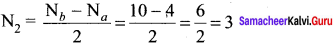
Bond order in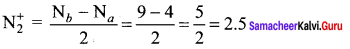
So, N2 is more stable than N2+. But bond length N2+ is greater than N2.
(b) NO+ & NO
Bond order of NO = 2.5
Bond order of NO+ = 3
Due to lesser bond order in NO, the bond length is greater than NO+ So, NO+ bond length is shorter than NO bond length.
Question 10.
Draw the MO diagram for acetylide ion C22- and calculate its bond order.
Answer:
Acetylide ion, C22- in acetylene

Electronic configuration of C2 ion is:
σ1s2 σ*1s2 σ1s2 π2px2 π2py2
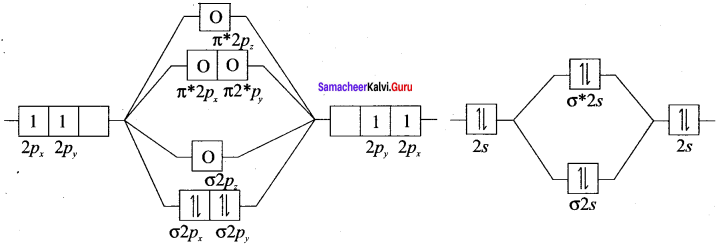
Bond order
Samacheer Kalvi 11th Chemistry Chemical Bonding Additional Questions Solved
Samacheer Kalvi 11th Chemistry Chemical Bonding 1 Mark Questions and Answer:
I. Choose the correct answer.
Question 1.
Which is the correct Lewis structure of Helium?

Answer:
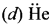
Solution:
Helium has only two electrons in its valence shell which is represented as a pair of dots (duplet).
Question 2.
The valency of C in CO32- is
a) 2
b) 3
c) 4
d) -3
Answer:
c) 4

Question 3.
In which one of the following molecule triple bond is present?
(a) O2
(b) H2
(c) CO2
(d) N2
Answer:
(d) N2
Solution:
N ≡ N
Question 4.
Which of the following is the lewis structure of water?
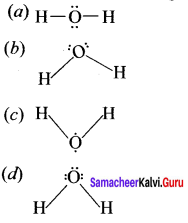
Answer:
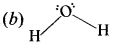
Question 5.
If the cyanide ion, the formal negative charge is on
a) C
b) N
c) Both C and N
d) Resonate between C and N
Answer:
b) N
Question 6.
Which one is the preferred structure of CO2?
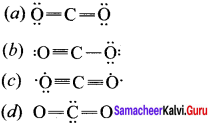
Answer:
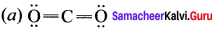
Question 7.
Which is the correct lewis structure of BF3?
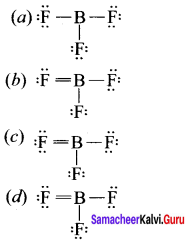
Answer:
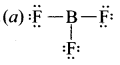
Question 8.
Statement I: In sulphur hexafluoride, the central atom has more than eight valence electrons.
Statement II: The central atom can accommodate additional electron pairs by using outer vacant d orbitals.
(a) Statements I and II are correct and statement II is the correct explanation of statement I.
(b) Statements I and II are correct but statement II is not the correct explanation of statement I.
(c) Statement I is correct but statement II is wrong.
(d) Statement I is wrong but statement II is correct.
Answer:
(a) Statements I and II are correct and statement II is the correct explanation of statement I.
Question 9.
Which of the following compounds has the maximum covalent nature?
a) LiCl
b) NaCl
c) KCl
d) CsCl
Answer:
a) LiCl
Question 10.
Which one of the following does not have electrovalent bond?
(a) KCI
(b) NaI
(c) MgO
(d) CCI4
Answer:
(d) CCI4

Question 11.
Which one of the following has an ionic bond?
(a) CO2
(b) CH4
(c) CaF2
(d) BeCI2
Answer:
(c) CaF2
Question 12.
During the formation of 1 mole of KCI crystal, the amount of energy released is ………….
(a) 418.81 kJ
(b) 348.56 kJ
(c) 718 kJ
(d) 70.25 kJ
Answer:
(c) 718 kJ
Question 13.
Point out an incorrect statement about resonance
a) Resonance structures should have equal energy
b) In resonance structures, the constituent atoms should be in the same position
c) In resonance structures, there should not be the same number of electron pairs
d) Resonance structures should differ only in the location of electrons around the constituent atoms
Answer:
c) In resonance structures, there should not be the same number of electron pairs
Question 14.
The distance between the nuclei of the two covalently bonded atoms is called …………….
(a) bond order
(b) bond length
(c) bond angle
(d) bond enthalpy
Answer:
(b) bond length
Question 15.
The length of a bond can be determined by ……………
(a) spectroscopic method
(b) x – ray diffraction method
(c) electron-diffraction method
(d) all the above
Answer:
(d) all the above
Question 16.
The value of carbon-carbon single bond length is ………..
(a) 1.43A
(b) 1.54Å
(c) 1.33A
(d) 1.20A
Answer:
(b) 1.54Å
Question 17.
For the formation of covalent bond, the difference in the value of electronegativities should be
a) Equal to or less than 1.7
b) More than 1.7
c) 1.7 or more
d) None of these
Answer:
a) Equal to or less than 1.7
Question 18.
The value of carbon-carbon triple bond length is ……………..
(a) 1.33A
(b) l.20Å
(C) 1.54A
(d) 1.43A
Answer:
(b) 1.20Å

Question 19.
Among the following which one has bond order as 3?
(a) N2
(b) O2
(c) HCHO
(d) CH4
Answer:
(a) N2
Question 20.
In a polar molecule, the ionic charge is 4.8 × 10-10 esu. If the interionic distance is 1 Å unit, then the dipole moment is
a) 41.8 debye
b) 4.18 debye
c) 4.8 debye
d) 0.48 debye
Answer:
c) 4.8 debye
Question 21.
Identify the molecule with bond order 1.
(a) N2
(b) O2
(c) H2
(d) C2H4
Answer:
(c) H2
Question 22.
Which one of the following has zero dipole moment?
(a) HF
(b) H2
(c) CO
(d) NO
Answer:
(b) H2
Question 23.
Which one of the following is called polar molecule?
(a) H3
(b) O2
(c) F2
(d) NO
Answer:
(d) NO

Question 24.
Statement I: CuCl is more covalent than NaCI.
Statement II: As compared to Na+. Cu+ is small and have 3s2 3p6 3d10 configuration and show greater polarisation.
(a) Statement I & II are correct and II is the correct explanation of I
(b) Statement I & II are correct but II is not the correct explanation of I
(c) Statement I & II are correct but II is wrong
(d) Statement I & II are wrong and II is the correct.
Answer:
(a) Statement I & II are correct and II is the correct explanation of I
Question 25.
Which of the following has the least dipole moment?
a) NF3
b) CO2
c) SO2
d) NH3
Answer:
b) CO2
Question 26.
Which one of the following has trigonal bipyramidal shape?
(a) SF6
(b) IF4+
(c) AsF5
(d) SF4
Answer:
(c) AsF5
Question 27.
Which one of the following does not have tetrahedral shape?
(a) NH4+
(b) ClO4–
(c) HCHO
(d) CH4
Answer:
(c) HCHO

Question 28.
Which one of the following has linear shape?
(a) O3
(b) CO32-
(c) NO3–
(d) BCl3
Answer:
(a) O3
Question 29.
Which is not true according to VBT?
a) A covalent bond is formed by the overlapping of orbitals with unpaired electrons of opposite spins
b) A covalent bond is formed by the overlapping of orbitals with unpaired electrons of same spins
c) The greater the extent of overlapping the stronger is the bond
d) Overlapping takes place only in the direction of maximum electron density of the orbital
Answer:
b) A covalent bond is formed by the overlapping of orbitals with unpaired electrons of same spins
Question 30.
Which one of the following has tetrahedral shape?
(a) HCHO
(b) BeCl2
(c) PbCl2
(d) CF2Cl2
Answer:
(d) CF2CI2
Question 31.
Which one of the following pair has T – shapcd structure?
(a) BrF3, CIF3
(b) SF4, IF4+
(c) PCl5, AsF5
(d) NH3, PF3
Answer:
(a) BrF3, CIF3
Question 32.
Which one of the following has pentagonal bipyramidal shape?
(a) XeF4
(b) XeOF4
(c) IF7
(d) IOF5
Answer:
(c) IF7

Question 33.
Which one of the following has linear shape?
(a) I3–
(b) ICI4–
(c) BrF5
(d) IOF5
Answer:
(a) I3–
Question 34.
Which one of the following is the correct increasing order of bond angle?
(a) H2O < CH4 < BF3 < BeCI2
(b) BeCI2 < BF3 < CH4 < H2O
(c) BF3 < CH4 < BeCI2 < H2O
(d) CH4 < BeCI2 < H2O < BF3
Answer:
(a) H2O < CH4 < BF3 < BeCI2
Question 35.
Which one of the following hybridisation takes place in the formation of BeCI2?
(a) sp2
(b) sp
(c) sp3
(d) dsp2
Answer:
(b) sp
Question 36.
Which hybridisation is possible in BF3?
(a) sp2
(b) sp
(c) sp3
(d) sp3d
Answer:
(a) sp2
Question 37.
Which one of the following has bond order as 2.5?
(a) O2
(b) NO
(c) CO
(d) H2
Answer:
(b) NO

Question 38.
Which one of the following is an electron deficient compound?
(a) Al2Cl6
(b) AlBr3
(c) SF6
(d) BF3
Answer:
(d) BF3
Question 39.
Apply the VSEPR model to XeF4, which of the following molecular shape is consistent with the model?
(a) Square planar
(b) Tetrahedral
(c) Square pyramidal
(d) Octahedral
Answer:
(a) Square planar
Question 40.
On the basis of molecular orbital theory, select the most appropriate option.
(a) The bond order of O2 is 2.5 and it is paramagnetic
(b) The bond order of O2 is 1.5 and it is paramagnetic
(c) The bond order of O2 is 2 and it is diamagnetic
(d) The bond order of O2 is 2 and it is paramagnetic
Answer:
(d) The bond order of O2 is 2 and it is paramagnetic

Question 41.
Which of the following molecule does not exist due to its zero bond order?
(a) H2–
(b) He2+
(c) He2
(d) H2+
Answer:
(c) He2
Question 42.
Which of the following molecules have bond order equal to 1?
(a) NO, HF, HCl, Li2, CO
(b) H2, Li2, HF, Br2, HCI
(c) Li2, B2, CO, NO, He2+
(d) B2, CO, He2+, NO, HF
Answer:
(b) H2, Li2, HF, Br2, HCI
Solution:
Bond order of He2+ = 0.5
Bond order of NO = 2.5
Bond order of CO = 3
Question 43.
Arrange the following molecules in decreasing order of bond length.
(a) O2 >O2– > O2+ > O22-
(b) O22- > O2– > O2 > O2–
(c) O22- > O2– > O2+ > O2
(d) O2+ > O2+ > O22- > O2
Answer:
(b) O22- > O2– > O2 > O2–
Solution: Since the bond length is inversely proportional to the bond order so option ‘b’ is correct.
Question 44.
Among the following which shows the maximum covalent character?
(a) MgCI2
(b) FeCI2
(c) SnCI2
(d) AICI3
Answer:
(d) AICI3
Question 45.
Which of the following has maximum number of lone pairs associated with Xe?
(a) XeF2
(b) XeO3
(c) XeF4
(d) XeF6
Answer:
(a) XeF2
Question 46.
During the formation of a chemical bond …………………
(a) energy decreases
(b) energy increases
(c) energy remains zero
(d) energy remains constant
Answer:
(a) energy decreases
Question 47.
Using MO theory, predict which of the following species has the shortest bond length?
(a) O2+
(b) O2–
(c) C22-
(d) O22+
Answer:
(d) O22+

Question 48.
Identify the incorrect statement regarding the molecule XeO4.
(a) XeO4 molecule is tetrahedral
(b) XeO4 molecule is square planar
(c) There are four pπ – dπ bonds
(d) There are four sp3 – p, s bonds
Answer:
(b) XeO4 molecule is square planar
Question 49.
Which of the following contains maximum number of lone pairs on the central atom?
(a) ClO3–
(b) XeF4
(c) SF4
(d) I3–
Answer:
(d) I3–
Question 50.
Which one of the following is a correct set?
(a) H2O, sp3, bent
(b) H2O, sp2, linear
(c) NH4+, dsp2, square planar
(d) CH4+, dsp2 tetrahedal
Answwer:
(a) H2O, sp3, bent
II. Match the following
Question 1.
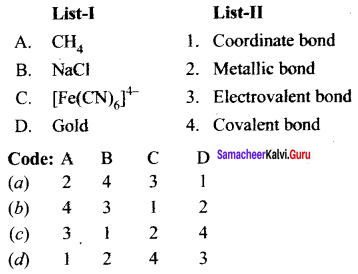
Answer:
(b) 4 3 1 2
Question 2.
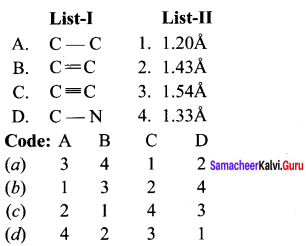
Answer:
(a) 3 4 1 2
Question 3.
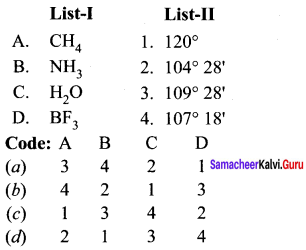
Answer:
(a) 3 4 2 1
Question 4.
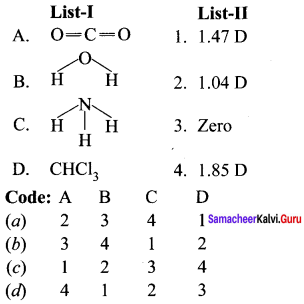
Answer:
(b) 3 4 1 2
Question 5.


Answer:
(c) 2 3 4 1
Question 6.
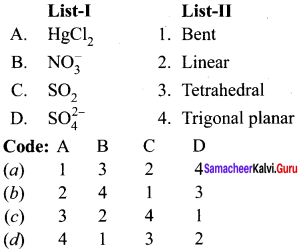
Answer:
(b) 2 4 1 3
Question 7.
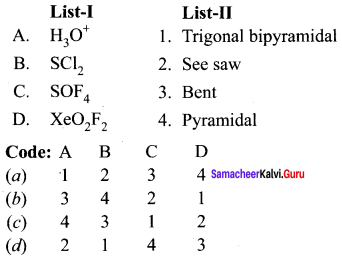
Answer:
(c) 4 3 1 2
Question 8.
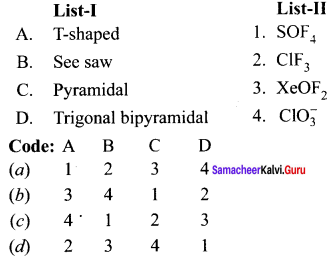
Answer:
(d) 2 3 4 1
Question 9.
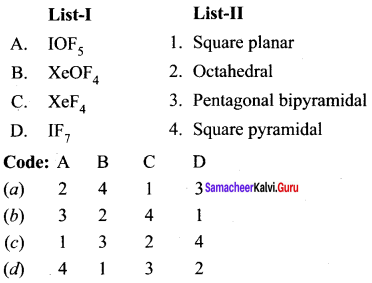
Answer:
(a) 2 4 1 3
Question 10.
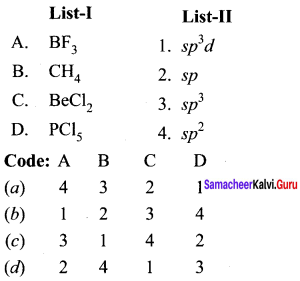
Answer:
(a) 4 3 2 1
Question 11.
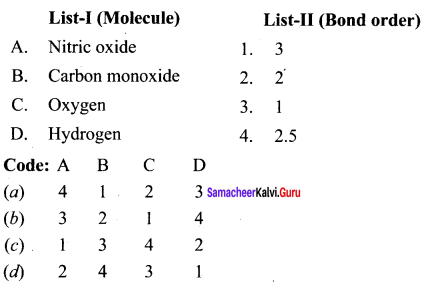
Answer:
(a) 4 1 2 3
III. Fill in the blanks.
Question 1.
The electrovalent bond is present in ………..
Answer:
NaCI
Solution:
Na+ cation and Cl– anion are held together by electrostatic attractive forces and this is called electrovalent bond.
Question 2.
The structure (or) shape of water molecule is …………
Answer:
inverted ‘V’ shape
Solution:

Question 3.
The structure of CO2 is ………..
Answer:
linear
Solution:
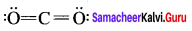
Question 4.
In the formation of a chemical bond between Na and C[, they attain the stable configuration of …………….
Answer:
Ne, Ar
Solution:
Na+: 1s2 2s2 2p6 = [Ne]
Cl–: 1s2 2s2 3s2 3p6 = [Ar]
Question 5.
The mutual sharing of one or more pair of electrons between the two combining atoms results in the formation of …………..
Answer:
Covalent bond
Question 6.
Formal charge of an atom can be calculated by the formula ………….
Answer:
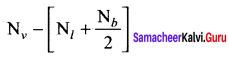
Question 7.
The formal charge on the carbon atom in the following structure
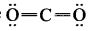 is …………………
is …………………
Answer:
zero
Solution:
Formal charge on carbon atom
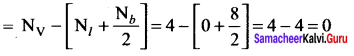
Question 8.
The formal charge on both oxygen atoms in the structure
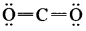 is …………..
is …………..
Answer:
0
Solution:
Formal charge on oxygen
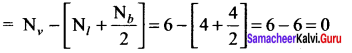
Question 9.
The formal charge on singly bonded oxygen atom in the structure
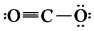 is …………..
is …………..
Answer:
-1
Solution:
Formal charge on singly bonded oxygen atom
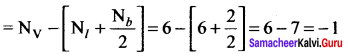
Question 10.
The formal charge on the triply bonded oxygen atom in the structure
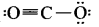 is …………
is …………
Answer:
+ 1
Solution:
Formal charge on triply bonded oxygen atom
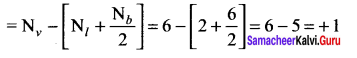
Question 11.
The complete transfer of one or more valence electron from one atom to another leads to the formation of ………….
Answer:
Ionic bond
Question 12.
The shape of the molecule is determined approximately by ……………
Answer:
bond angle
Question 13.
The unit of bond enthalpy is …………..
Answer:
kJ mol-1
Question 14.
The electronegativity of hydrogen and fluorine on Pauling’s scale are …………..
Answer:
2.1 and 4

Question 15.
The unit of dipole moment is ……………
Answer:
Coulomb-1 m2
Question 16.
The dipole moment of CO2 is ……………
Answer:
0
Solution:
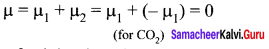
Question 17.
The shape of sulphur hexafluoride is …………
Answer:
Octahedral
Question 18.
The type of hybridisation takes place in methane is ………………
Answer:
sp3
Question 19.
The type of hybridisation takes place in SF6 is …………..
Answer:
sp3d2
Question 20.
The number of lone pair of electrons on C – atom present in CO2 are …………
Answer:
4
Question 21.
In SF6, the bond angle is …………….
Answer:
900
Question 22.
The ions have noble gas electronic configuration was suggested by ……………….
Answer:
Kossel
Question 23.
Tetrachlorornethane is a molecule …………..
Answer:
non-polar
Question 24.
In C2H4, type of bonds present are ……………
Answer:
Covalent bonds only
Question 25.
Molecule with bond of shape trigonal pyramid is …………..
Answer:
BF3
Question 26.
When magnesium reacts with oxygen, nature of bond formed is …………….
Answer:
ionic

Question 27.
The number of lone pair of electrons in water molecule is …………..
Answer:
2
Question 28.
Double bonds as compared to single bonds are ……………….
Answer:
Shorter
Question 29.
Number of chlorine atoms which form equatorial bonds in PCI5 molecule are/is ………..
Answer:
3
Question 30.
The hybridisation of B in BF3 is ……………
Answer:
sp2
Question 31.
Bond order of O2, F3, N2 respectively are ……………
Answer:
2, 1, 3
Question 32.
Hybridisation which takes place in acetylene is …………….
Answer:
sp
Question 33.
Bond order of O2, F2, N2 respectively are ………….
Answer:
2, 1,3
Question 34.
Hybridisation which takes place in acetylene is …………..
Answer:
sp

Question 35.
The hybridisation of orbitais of N atom in NO3–, NO3+ and NH4+ are respectively ……………….
Answer:
sp2, sp, sp3
Question 36.
Malleability and ductility of metals can be accounted due to the capacity of layers of …………………. to slide over one another.
Answer:
metal ions
Question 37.
For a stable molecule, the value of bond order must be ……………
Answer:
positive
Question 38.
In acetylene molecule between the carbon atoms, there are ………….. σ and ……………. bonds.
Answer:
one, two
IV. Choose the odd one out.
Question 1.
(a) Hydrogen
(b) Chlorine
(c) Neon
(d) Argon
Answer:
(c) Neon. It is monoatomic whereas others are diatomic.
Question 2.
(a) NaCl
(b) CO2
(c) LiF
(d) MgO
Answer:
(b) CO2. It contains covalent bond whereas others have ionic bond.
Question 3.
(a) Methane
(b) Ceasium chloride
(c) Ammonia
(d) Boron trifluoride
Answer:
(b) Ceasium chloride. It is an ionic compound whereas others are covalent compounds.

Question 4.
(a) H2
(b) O2
(c) Cl2
(d) F2
Answer:
(b) O2. It’s bond order is 2 whereas in others bond order is 1.
Question 5.
(a) BeCI2
(b) CS2
(c) BF3
(d) HCN
Answer:
(c) BF3. It is AB3 type whereas others are AB2 type.
Question 6.
(a) XeO2F2
(b) PCI5
(c) AsF5
(d) SOF4
Answer:
(a) XeO2F2. It is AB4L type whereas others are AB5 type.
V. Choose the correct pair.
Question 1.
(a) NaCI – ionic compound
(b) NH3 – coordinate compound
(c) BF3 – ionic compound
(d) H2 – ionic compound
Answer:
(a) NaCl – ionic compound
Question 2.
(a) O2 – Bond order 3
(b) H2 – Bond order 2
(c) N2 – Bond order 3
(d) Cl2 – Bond order 2
Answer:
(c) N2 – Bond order 3
[N = N] Bond order is 3.
Question 3.
(a) CH4 – ionic bond
(b) BF3 – dative bond
(c) NH3 – metallic bond
(d) CCI4 – covalent bond
Answer:
(d) CCI4 – covalent bond

Question 4.
(a) CH4 – 107° 18’
(b) H2O – 109°28’
(c) NH3 – 104°35’
(d) BF3 – 120°
Answer:
(d) BF3 – 120°
Question 5.
(a) AB3 – Linear
(b) AB3 – V-shape(or)bent
(c) AB4 – Trigonal planar
(d) AB5 – T-shape
Answer:
(a) AB3 – Linear
VI. Choose the incorrect pair.
Question 1.
(a) CS2 – Linear
(b) BF1 – Trigonal planar
(c) CH4 – T-shape
(d)NH3 – Pyramidal
Answer:
(c) CH4 – T-shape
Question 2.
(a) AB3 – Trigonal planar
(b) AB3L2 – T-shape
(c) AB5 – Trigonal bipyramidal
(d) AB3L – Bent
Answer:
(a) AB3L: Bent.
Actually, AB3L is pyramidal.
Question 3.
(a) AB7 – IF7
(b) AB4L2 – ICI4–
(c) AB6 – XeOF4
(d) AB5L – IF5
Ans.
(c) AB6 – XeOF4
Actually XeOF4 is AB5L type.

Question 4.
(a) Fluorine – Bond order 1
(b) Oxygen – Bond order 2
(c) Nitrogen – Bond order 2
(d) Cyanide – Bond order 3
Answer:
(c) Nitrogen – Bond order 2.
Actually N = N bond order is 3.
Question 5.
(a) CH4 – sp3
(b) PCI5 – sp3d
(c) BeCl2 – sp
(d) BF3 – sp3d2
Answer:
(d) BF3 – sp3d2
Actually BF4 is sp2 hybridised.
VII. Assertion and Reason.
Question 1.
Assertion (A): Diatomic molecules such as H2, O2, F2 are non-polar molecules.
Reason (R): H2, O2, F2 have zero dipole moment.
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
(b) Both (A) and (R) are correct but (R) is not the correct explanation of (A).
(c) (A) is correct but (R) is wrong.
(d) (A) is wrong but (R) is correct.
Answer:
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
Question 2.
Assertion (A): HF, HCl, CO and No are polar molecules.
Reason (R): They have non zero dipole moments and so they are polar molecules.
(a) Both (A) and (R) are correct and (R) is the correct explanation of(A).
(b) Both (A) and (R) are correct but (R) is not the correct explanation of(A).
(c) (A) is correct but (R) is wrong.
(d) (A) is wrong but (R) is correct.
Answer:
(a) Both (A) and (R) are correct and (R) is the correct explanation of(A).
Question 3.
Assertion (A): H2, Li2, C2, N2 are diamagnetic.
Reason (R): All have no unpaired electrons and so they are diamagnetic.
(a) Both (A) and (R) are correct and (R) is the correct explanation of(A).
(b) Both (A) and (R) are correct but (R) is not the correct explanation of(A).
(c) (A) is correct but (R) is wrong.
(d) (A) is wrong but (R) is correct.
Answer:
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
Question 4.
Assertion (A): B2, O2, NO are paramagnetic in nature.
Reason (R): They have unpaired electrons and are paramagnetic.
(a) Both (A) and (R) are correct but (R) is not the correct explanation of(A).
(b) Both (A) and (R) are correct and (R) is the correct explanation of(A).
(c) (A) is correct but (R) is wrong.
(d) (A) is wrong but (R) is correct.
Answer:
(b) Both (A) and (R) are correct and (R) is the correct explanation of (A).

Question 5.
Assertion (A): Metals have high thermal conductivity.
Reason (R): Absence of bond gap is the main reason for high thermal conductivity.
(a) Both (A) and (R) are correct and (R) is the correct explanation of(A).
(b) Both (A) and (R) are correct but (R) is not the correct explanation of(A).
(c) (A) is correct but (R) is wrong.
(d) (A) is wrong but (R) is correct.
Answer:
(b) Both (A) and (R) are correct but (R) is not the correct explanation of(A).
Question 6.
Assertion (A): Metals have high thermal conductivity.
Reason (R): Due to the thermal excitation of many electrons from the valence band to the conductance band, metals have high thermal conductivity.
(a) Both (A) and (R) are correct and (R) is the correct explanation of(A).
(b) Both (A) and (R) are correct but (R) is not the correct explanation of(A).
(c) (A) is wrong but (R) is correct.
(d) (A) is correct but (R) is wrong.
Answer:
(a) Both (A) and (R) are correct and (R) is the correct explanation of(A).
VIII. Choose the correct statement.
Question 1.
(a) The metallic luster is due to reflection of light by the electron cloud.
(b) Metals have low inciting point and low boiling point.
(c) Metals have low thermal conductivity.
(d) Electrical conductivity of metals is low.
Answer:
(a) The metallic luster is due to the reflection of light by the electron cloud.
Question 2.
(a) NO molecules are diamagnetic
(b) O2 molecules are paramagnetic
(c) N2 molecules are paramagnetic
(d) Li2 molecules are paramagnetic
Answer:
(b) O2 molecules is paramagnetic

Question 3.
(a) BeCl2 undergoes sp3 hybridisation
(b) 8F3 undergoes sp3d hybridisation
(c) CH4 undergoes sp3d2 hybridisation
(d) PCl5 undergoes sp3d hybridisation
Answer:
(d) PCl5 undergoes sp3d hybridisation
Samacheer Kalvi 11th Chemistry Chemical Bonding 2 Mark Questions and Answers
I. Write a brief answer to the following questions.
Question 1.
What are chemical bonds?
Answer:
The interatomic attractive forces which hold the constituent atoms/ions together in a molecule are called chemical bonds.

Question 2.
State octet rule.
Answer:
The atoms transfer or share electrons so that all the atoms involved in chemical bonding obtain eight electrons in their outer shell (valence shell). It is called the octet rule.
Question 3.
What is meant by a covalent bond?
Answer:
The mutual sharing of one or more pair of electrons between two combining atoms results in the formation of a chemical bond called a covalent bond.
Question 4.
Draw the lewis structure of
- H2O
- SO3.
Answer:
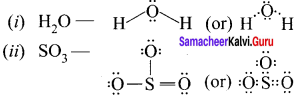
Question 5.
Calculate the formal charge on the carbon atom and oxygen atom in the structure
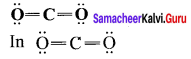
Answer:
Formal charge on carbon
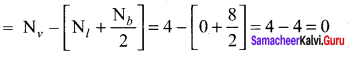
Formal charge on oxygen
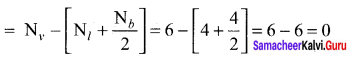
Question 6.
Calculate the formal charge on the carbon atom and oxygen atom in the structure
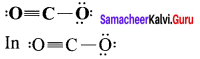
Answer:
Formal charge on carbon
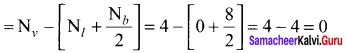
Formal charge on singly bonded oxygen
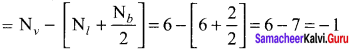
Formal charge on triply bonded oxygen
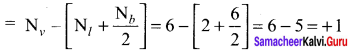
Question 7.
Among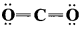 and
and 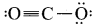 which is a preferable structure for CO2 molecule why?
which is a preferable structure for CO2 molecule why?
Answer:
Structure I of 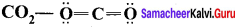
Formal charge on carbon
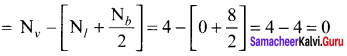
Formal charge on oxygen
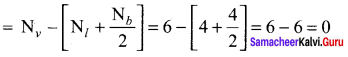
Structure II of 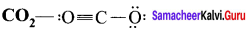
Formal charge on carbon
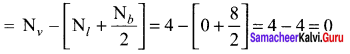
Formal charge on singly bonded oxygen
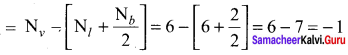
Formal charge on triply bonded oxygen
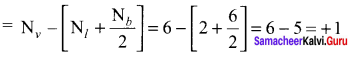
A structure in which all formal charges are zero is preferred over the one with non – zero charges. In case of CO2 structure, structure I is preferred over structure II as it has zero formal charge for all the atoms.
Question 8.
Draw the lewis structures of a few molecules containing odd electrons.
Answer:
Few molecules have a central atom with an odd number of valence electrons. For example, in nitrogen dioxide and nitric oxide all the atoms does not have octet configuration.
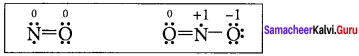
Question 9.
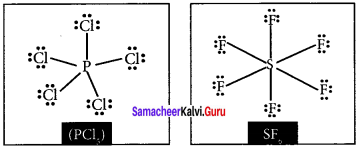
Draw the lewis structure of PCl5 and SF6
Answer:
Question 10.
Define bond length.
Answer:
The distance between the nuclei of two covalently bonded atoms is called bond length. For e.g., in a covalent molecule A – B. the bond length is equal to the sum of the radii of bonded atoms. i.e., rA + rß = bond length.
Question 11.
Prove that bond order is inversely proportional to bond length.
Answer:
1. Bond order ∝ 
2. An example for illustrating the above equation is Carbon-carbon single bond length (I .54Å) is longer than the carbon-carbon double bond length (1 .34Å) and the carbon- carbon triple bond length (1 .20Å).
Question 12.
Define Bond angle.
Answer:
Covalent bonds are directional in nature and are oriented in specific direction in space. This directional nature creates a fixed angle between two covalent bonds in a molecule and this angle is termed as bond angle.
Question 13.
Define Resonance.
Answer:
The similar structures in which the relative position of the atoms are same but they differ in the position of bonding and lone pair of electrons are called resonance structures and this phenomenon is called resonance.

Question 14.
What are polar and non-polar molecules?
Answer:
1. Diatomic molecules such as H2, O2, F2 have zero dipole moment and are called non polar molecules.
2. Molecules such as HF, HCl, CO, NO have non zero dipole moment values and are called polar molecules.
Question 15.
What is meant by polarisaion?
Answer:
The ability of a cation to polarise an anion is called its polarising ability and the tendency of the anion to get polarised is called its polarisability. This phenomenon is known as polarisation.
Question 16.
Among NaCI, MgCI2 and AICI3 which shows more covalent character? Why?
Answer:
Among, the ionic compounds NaCI, MgCl2 and AICI3 the charge of the cation increases in the order Na+ < Mg2+ < Al3+, thus the covalent character also follows the same order NaCl < MgCI2 < AlCI3. So AICI3 shows more covalent character.
Question 17.
Lithium chloride is more covalent than sodium chloride. Justify this statement.
Answer:
1. The smaller cation and larger anion shows greater covalent character due to greater extent of polarisation.
2. The size of Li+ ion is smaller than Na+ ion and hence the polarising power of Li+ ion is more. So lithium chloride is more covalent than sodium chloride.
Question 18.
Lithium iodide is more covalent than Lithium chloride. Give reason.
Answer:
Lithium iodide is more covalent than Lithium chloride as the size of I– ion is larger than Cl– ion. Hence I– ion will be more polarised than Cl– ion by the cation Li+. So LiI is more covalent than LiCl.
Question 19.
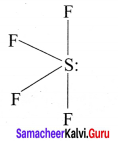
Draw the structure of AB4L and AB3L2 type of molecules with example.
Answer:
AB4L:
Bond pairs = 4
Lone pair = 1
Shape = see saw
e.g., SF4 Strucutre
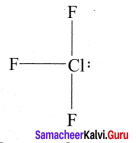
AB3L2:
Bond pairs = 3
Lone pair = 2
Shape = T shaped
e.g., CIF3 Strucutre
Question 20.
Draw the structure of AB4L2 and AB7 type of molecules with example
Answer:
1. AB4L2:
Bond pairs = 4
Lone pairs = 2
Shape = Square planare.g., XeF4
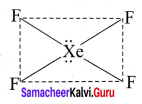
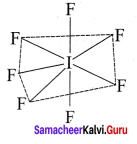
2. AB7:
Bond pairs = 7
Lone pairs = Nil
Shape = pentagonal bipyramidal
e.g., IF7
Question 21.
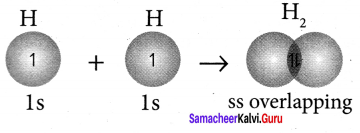
Explain the bond formation of hydrogen molecule.
Answer:
1. Electronic configuration of hydrogen atom is 1s1.
2. During the formation of H2 molecule, the 1 s orbitais of two hydrogen atoms containing one unpaired electron with opposite spin overlaps with each other along the internuclear axis.
This overlap is called s – s overlap. Such axial overlap results in the formation of a sigma (σ) covalent bond.
Question 22.
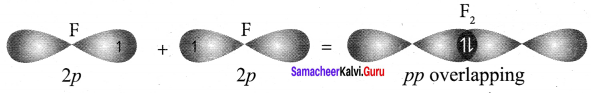
Explain the bond formation of fluorine molecule.
Answer:
1. Valence shell electronic confIguration of fluorine atom is 2s2 2px2 2py2 2pz1
2. When the half filled pz orbitais of two fluorine atoms overlap along the z – axis, a σ covalent bond is formed between them.
Question 23.
How is HF molecule formed?
Answer:
- Electronic configuration of hydrogen atom is 1s1.
- Valence shell electronic configuration of fluorine atom is 2s2 2px2 2py2 2pz1.
- When half filled is orbital of hydrogen linearly overlaps with a halt filled 2pz orbital of fluorine, a σ covalent bond is formed between hydrogen and fluorine.
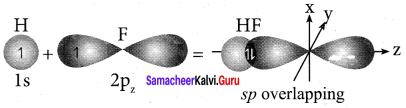
Question 24.
What is meant by metallic bond?
Answer:
The forces that keep the atoms of the metal so closely in a metallic crystal constitute what is generally known as the metallic bond.
Question 25.
Why metallic bonding is referred as electronic bonding?
Answer:
1. Metallic crystals are an assemblage of positive ions immersed in a gas of free electrons. The free electrons are due to the ionisation of the valence electrons of the atom of the metal.
2. As the valence electrons of the atoms are freely shared by all the ions in the crystal, the metallic bonding is also referred to as electronic bonding.
Question 26.
Metals have high density. Give reason.
Answer:
The electrostatic attraction between the metal ions and the free electrons yields a three dimensional close packed crystal with a large number of nearest metal ions. So metals have high density.

Question 27.
Metals are ductile in nature. why?
Answer:
In the close packed structure of metallic crystal. it contains many slip planes along which movement can occur during mechanical loading, so the metal acquires ductility.
Question 28.
Give reason behind the lustrous nature, high melting point and boiling point of metals?
Answer:
1. The metallic lustre is due to the reflection of light by the electron cloud.
2. As the metallic bond is strong enough. the metal atoms are reluctant to break apart into a liquid or gas, so the metals have high melting and boiling points.
Question 29.
Metals are very good electrical conductors. Why?
Answer:
1. The bonding in metal is explained by molecular orbital theory. As per this theory. the atomic orbitals of large number of atoms in a crystal overlap to form numerous bonding and anti-bonding molecular orbitals without any band gap.
2. The bonding molecular orbitals are completely filled with an electron pair in each, and the anti-bonding molecular orbitals are empty.
3. Absence of band gap accounts for high electrical conductivity of metals.
Question 30.
Metals have high thermal conductivity. Give reason.
Answer:
High thermal conductivity of metals is due to thermal excitation of many electrons from the valence band to the conduction band.

Question 31.
Except Cu, Ag and Au, most metals are black. Why?
Answer:
Most metals are black except copper, silver and gold. It is due to the absorption of light of all wavelengths. Absorption of light of all wavelengths is due to the absence of band gap in metals.
Question 32.
Write the favourable factors for the formation of ionic bond.
Answer:
- Low ionisation enthalpy of metal atoms.
- High electron gain enthalpy of non-metal atoms.
- High lattice enthalpy of compound formed.
Question 33.
Although geometries of NH3 and H2O molecules are distorted tetrahedral, bond angle in water is less than that of ammonia. Discuss.
Answer:
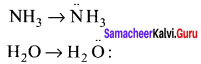
Because of two lone pair of electrons on O – atom, repulsion on bond pairs is greater in H2O in comparison to NH3. Thus, the bond angle is less in H2O molecules.
Question 34.
Write the significance/applications of dipole moment.
Answer:
- In predicting the nature of the molecules: Molecules with specific dipole moments are polar in nature and those with zero dipole moments are non-polar in nature.
- In the determination of shapes of molecules.
- In calculating the percentage of ionic character.

Question 35.
Although both CO2 and H2O are triatomic molecules, the shape of H2O molecule is bent while that of CO2 is linear. Explain this on the basis of dipole moment.
Answer:
In CO2, there are two C = O bonds. Each C = O bond is a polar bond. The net dipole moment of CO2 moleculc is zero. This is possible only if CO2 is a linear molecule. (O = C = O). The bond dipoles of two C = O bonds cancels the dipole moment of each other.
Whereas, H2O molecule has a net dipole moment (1.84 D). H2O molecule has a bent structure because here the O – H bonds are oriented at an angle of 104.5° and do not cancel the bond dipole moments of each other.
Question 36.
What is the total number of sigma and pi bonds in the following molecules?
- C2H2
- C2H4
Answer:
1. H – C = C – H
Sigma bond = 3
π bond = 2
2.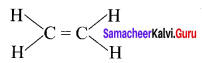
Sigma bond = 5
π bond = 1
Question 37.
Use molecular orbital theory to explain why the Be2 molecule does not exist
Answer:
E.C of Be = 1 s2 2s2
M.O.E.C of Be2 = σ1s2 σ2s2 σ*2s2
Bond order = \(\frac { 1 }{ 2 }\) (4 – 4) = 0
Hence, Be2

Question 38.
Compare the relative stabililty of the following species and indicate their magnetic properties O2, O2+, O2– (superoxide), O22-(peroxide)
Answer:
O2 – Bond order = 2 paramagnetic
O2+ – Bond order = 2.5, paramagnetic
O2– – Bond order = 1.5, paramagnetic
O22- – Bond order = 1, diagmagnetic
Order of relative stability is:
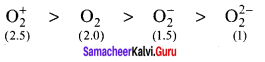
Question 39.
Account for the following:
- Water is a liquid while H2S is a gas
- NH3 has higher boiling point than PH3.
Answer:
- In case of water, hydrogen bonding causes association of the H2O molecules. There is no such hydrogen bonding in H2S, that is why it is a gas.
- There is hydrogen bonding in NH3 but not in PH3.
Question 40.
Why B2 is paramagnetic in nature while C2 is not?
Answer:
The molecular orbital electronic configuration of both B2 and C2 are:
B2:
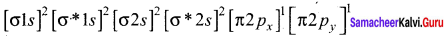
C2:
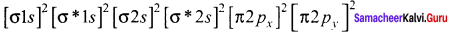
Since, B2 has two unpaired electrons, therefore, B2 is paramagnetic C2 has no unpaired electron, therefore, C2 is diamagnetic.
Samacheer Kalvi 11th Chemistry Chemical Bonding 3 Mark Questions and Answers
Question 1.
Draw the lewis structure of
- Nitrogen
- Carbon
- Oxygen
Answer:

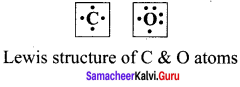
Question 2.
Draw the lewis structure of
- Ammonia
- Methane
- Dinitrogen pentoxide.
Answer:
Lewis dot structures of Molecules
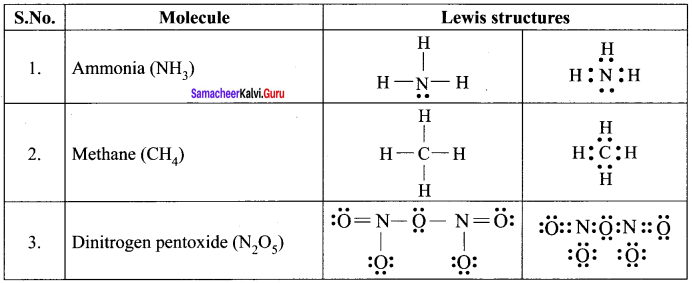
Question 3.
Calculate the bond enthalpy of OH bond in water.
Answer:
1. In the case of polyatomic molecules with two or more same bond types, the arithmetic mean of the bond energy value of the same type of bonds is considered as average bond enthalpy.
2. For e.g., in water, there are two OH bonds present and the energy needed to break them are not same.
3. H2O(g) → H(g) + OH(g)
∆H1 = 502 kJ mol-1
OH(g) → H(g) + O(g)
∆H = 427 kJ mol
The average bond enthalpy of OH bond in water = \(\frac { 502 + 427 }{ 2 }\) = 464.5 kJ mol-1
Question 4.
Explain how the ionic character in a covalent bond is related to electronegativity?
Answer:
1. The extent of ionic character in a covalent bond can be related to the electronegativity difference of the bonded atoms.
2. In a typical polar molecule Aδ- – Bδ+ the electronegativity difference (XA – XB) can be used to predict the percentage of the ionic character as follows
3. If the electronegativity difference XA – XB is equal to 1.7, then the bond A – B has 50% ionic character.
4. If it is greater than 1.7, then the bond XA – XB has more than 50% ionic character.
5. If it is greater than 1.7, then the bond A – B has more than 50% ionic character.
6. If it is lesser than 1.7, then the bond A – B has less than 50% ionic character.

Question 5.
CuCI is more covalent than NaCl. Give reason.
Answer:
1. Cations having ns2np6nd10 configuration show greater polansing power than the cations with ns2 np6 configuration. Hence they show greater covalent character.
2. CuCI is more covalent than NaCI. As compared to Na+ (1.13Å), Cu+(0.6Å)is small and has 3s23p63d10 configuration.
3. Electronic configuration of Cu+: [Ar] 3d10
Electronic configuration of Na+: [He] 2s22p6
So CuCI is more covalent than NaCI
Question 6.
Draw the structure of AB2, AB3, AB3L type of molecules with example.
Answer:
1. AB2
Number of bond pairs = 2
Shape = Linear
Example – BeCl2

2. AB3
Number of bond pairs = 3
Shape = Tirgonal planar
Example – BF3
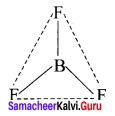
3. AB2L
Number of bond pairs = 2
Number of lone pairs = 1
Shape = Bent (or) inverted V shape
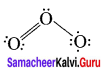
Example – O3
Question 7.
Give example and structure of
- AB3L
- AB5
- AB2L2
type of molecules with example.
Answer:
1. AB3L
Number of bond pairs = 3
Number of lone pairs = 1
Shape = Pyramidal
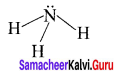
Example – NH3
2. AB5
Number of bond pairs = 5
Number of lone pairs = 0
Shape = Trigonal bipyramidal
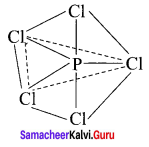
Example = PCIC5
3. AB2L2
Number of bond pairs = 2
Number of lone pairs = 2
Shape = Bent
Example – H2O
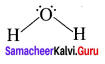
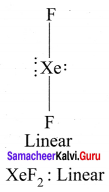
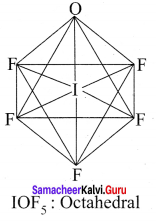
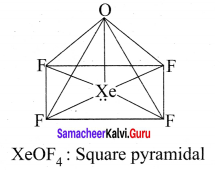
Question 8.
Draw the shape of
- XeF2
- IOF5
- XeOF4
Answer:
1. XeF2
2. IOF5
3. XeOF4
Question 9.
Explain the bonding in oxygen molecule.
Answer:
1. Valence shell electronic configuration of oxygen atom is
2s2 2px2 2py12pz1

2. When the half filled pz orbitaIs of two oxygen atoms overlap along the z – axis a σ covalent bond is formed between them. Other two hail filled py orbitais of two oxygen atoms overlap laterally to form a π – cova1ent bond between the oxygen atoms.
3. Thus in oxygen molecule, two oxygen atoms are connected by two covalent bonds (double bond). The other two pair of electrons present in the 2s and 2px orbital do not involve in bonding and remains as lone pair on the respective oxygen atoms.
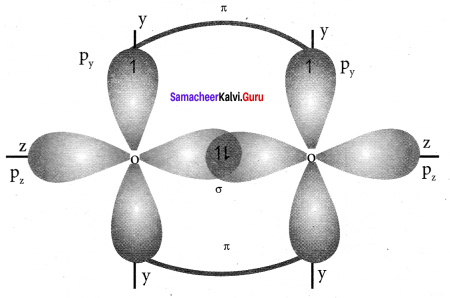
Question 10.
Explain about the molecular orbital diagram of hydrogen molecule.
Answer:
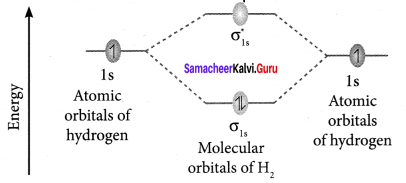
- Electronic configuration of H atom 1s1
- Electronic configuration of H, molecule – σ1s1
Bond order 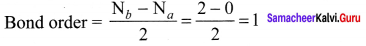
- Molecule (H2) has no unpaired electrons, hence it is diamagnetic.
Question 11.
Draw and explain the M.O. diagram of lithium molecule.
Answer:
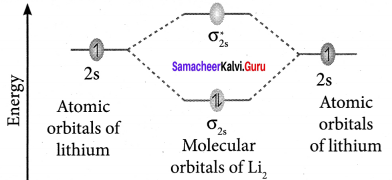
- Electronic configuration of Li atom – 1s1
- Electronic configuration of Li2 molecule is ais2 σ*1s2 σ*1s2 σs2
- Bondorder = Nb – Nb/2 = 4 – 2/2
- Li2 molecule has no unpaired electrons, hence it is diamagnetic.
Question 12.
Draw and explain the M.O. diagram of Boron molecule.
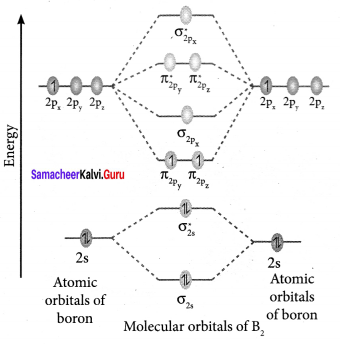
Answer:
- Electronic configuration of B = 1s2 2s2 2p3
- Electronic configuration of B, = σ1s2 σ*1s2σ2s2 σ*2s2 π2px1 π 2pz1
- Bond order
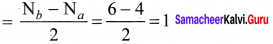
- B2 molecule has two unpaired electrons hence it is paramagnetic.
Question 13.
Draw and explain the molecular orbital diagram of carbon molecule.
Answer:
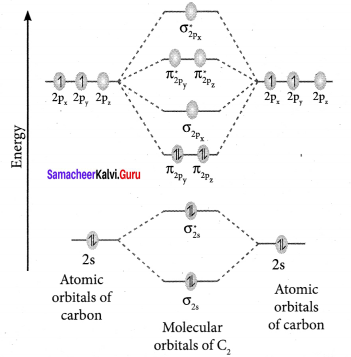
- Electronic configuration of C atom – 1s2 2s2 2p2
- Electronic configuration of C2 molecule is σ1s2 σ*1s2 σ*2s2 σ*2s2 π 2px2 π 2py2
- Bond order
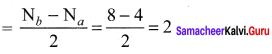
Question 14.
Write Lewis dot symbols for atoms of the following elements: Mgq Naq B O, N, Br.
Answer:

Question 15.
write Lewis symbols for the following atoms and ions: S and S2-; Al and Al3+; H and OH–
Answer:
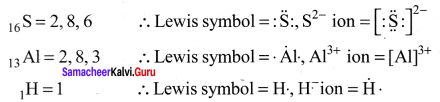
Question 16.
Draw the Lewis structures for the following molecules and ions H2S, SiCl4, BeF2, CO32-, HCOOH
Answer:
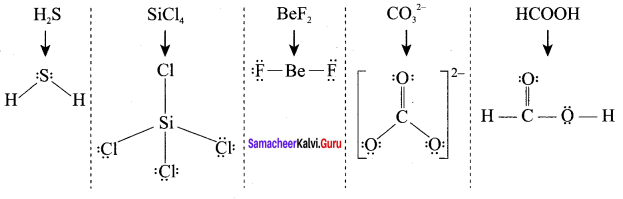
Question 17.
Define Octet rule. Write its significance and limitations.
Answer:
Octet rule:
Atoms of elements combine with each other in order to complete their respective octet so as to acquire the stable nearest noble gas configuration.
Significance:
It helps to explain why dilfferent atoms combine with each other to form ionic compounds or covalent compounds.
Limitations of Octet rule:
1. According to octet rule, atoms take part in chemical combination to achieve the configuration of nearest noble gas elements.
However, some of noble gas elements like Xenon have formed compounds with fluorine and oxygen. For example: XeF2, XeF4, XeO3 etc. Therefore, validity of the octet rule has been challenged.
2. This theory does not account for the shapes of molecules.
Question 18.
Write the resonance structure for SO3, NO2 and NO3
Answer:
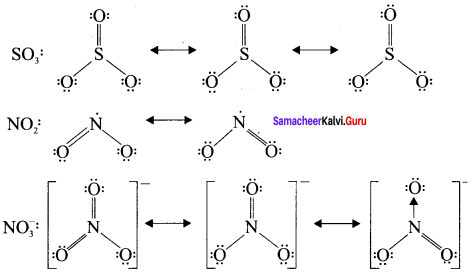
Question 19.
What do you understand by bond pairs and lone pairs of electrons? Illustrate by giving one example of each type.
Answer:
The electron pair involved in sharing between two atoms during covalent bonding is called shared pair or bond pair. At the same time, the electron pair which is not involved in sharing is called lone pair of electrons.
For example, in 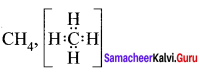
there are only 4 bond pairs, but in 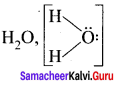 there are two bond pairs and two lone pairs.
there are two bond pairs and two lone pairs.
Question 20.
Distinguish between a sigma bond and a pi bond
Answer:
Sigma (σ) Bond
- σ – bond is formed by the axial overlap of the atomic orbitais.
- The bond is quite strong.
- Only one lobe ofthep-orbitals is involved in the overlap.
- Electron cloud of the molecular orbital is symmetrkal around the internuclear axis.
Pi (π) Bond
- π – bonnd is formed by the sidewise overlap of atomic orbitais.
- It is comparatively a weaker bond.
- Both lobes of the p-orbitais are involved in the overlap.
- The electron cloud is not symmetrical.

Question 21.
Write the Important conditions required for the linear combination of atomic orbitals to form molecular orbitals.
Answer:
1. The combining atomic orbitals should have comparable energies. For example, is orbital of one atom can combine with 1s atomic orbital of another atom, 2s orbitai can combine with 2s orbital and so on.
2. The combining atomic orbitals must have proper orientations so that they are able to overlap to a considerable extent.
3. The extent of overlapping should be large.
Question 22.
What are Lewis structures? Write the Lewis structure of H2, BeF2 and H2O.
Answer:
The outer shell electrons are shown as dots surrounding the symbol of the atom. These symbols are known as Lewis symbols or Lewis structures. The Lewis structure of
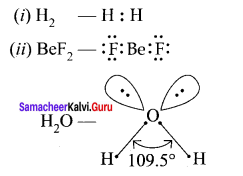
Question 23.
What are the main postulates of Valence Shell Electron Pair Repulsion (VSEPR) theory?
Answer:
- The shape of a molecule depends upon the no. of electron pairs around the central atom.
- There is a repulsive force between the electron pairs, which tend to repel one another.
- The electron pairs in space tend to occupy such positions that they arc at maximum distance, so that the repulsive force will be minimum.
- A multiple bond is treated as lilt is a single bond and the remaining electron pairs which constitute the bond may be regarded as single super pair.
Question 24.
Apart from tetrahedral geometry, another possible geometry for CH4 is square planar with four H atoms at the corners of the square and C atom at its centre. Explain why CH4 is not square planar?
Answer:
Electronic configuration of carbon atom: C: σ1s22s22p2.
in the excited state, the orbital picture of carbon can be represented as:

Hence, carbon atom undergoes sp3 hybridisation in CH4 molecule and takes tetrahedral shape.
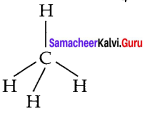
For a square planar shape, the hybridisation of the central atom has to be dsp3. However, an atom of carbon does not have d – orbitals to undergo dsp3 hybridisation. Hence, the structure of CH4 is tetrahedral.
Question 25.
Explain why BeH2 molecule has a zero dipole moment although the Be – H bonds are polar.
Answer:
The Lewis structure for BeH2 molecule is as follows: . There is no lone pair at the central atom (Be) and there are two bond pairs. Hence, BeH2, is of the type AB2. It has a linear structure.
. There is no lone pair at the central atom (Be) and there are two bond pairs. Hence, BeH2, is of the type AB2. It has a linear structure.
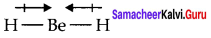
Dipole moments of each Be – H bond are equal and opposite in direction. Therefore, they nullify each other. Hence, BeH2 has a net zero dipole moment.
IV. Answer the following questions in detail:
Question 1.
Explain about Kossel-Lewis’s approach to chemical bonding.
Answer:
1. Kossel and Lewis’s approach to chemical bonding is based on the inertness of the noble gases which have little or no tendency to combine with other atoms.
2. They proposed that noble gases are stable due to their completely filled outer electronic configuration.
3. Elements other than noble gases try to attain the completely filled outer electronic configuration by losing, gaining or sharing one or more electrons from their outer shell.
4. For e.g., sodium loses one electron to form Na ion and chlorine accepts that electron to give chloride ion, Cl–. These two ions are held together by electrostatic attractive forces, a bond known as an electrovalent bond.
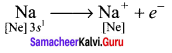
5. In diatomic molecules such as nitrogen and oxygen, they achieve the stable noble gas electronic configuration by mutual sharing of electrons.
6. Lewis introduced a scheme to represent the chemical bond and the electrons present in the outer shell of the atom called Lewis dot structure.

7. For example, the electronic configuration of nitrogen is 1s22s22p3. It has 5 electrons in its outer shell. The lewis structure of nitrogen is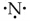
8. In N, molecule, equal sharing of 3 electrons from each nitrogen atom takes place as fol lows
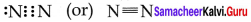
Question 2.
What is meant by covalent bond?
Explain the covalent bonding in H2, O2, N2.
Answer:
1. Mutual sharing of one or more pair of electrons between two combining atoms results in the formation of a chemical bond called a covalent bond.
2. If two atoms share just one pair of electron, a single covalent bond is formed as in the çase of hydrogen molecule (H2).
3. If two or three electron pairs are shared benveen the two combining atoms, then the covalent bond is called double bond and triple bond respectively, as in the case of O2 and N2 molecules respectively.
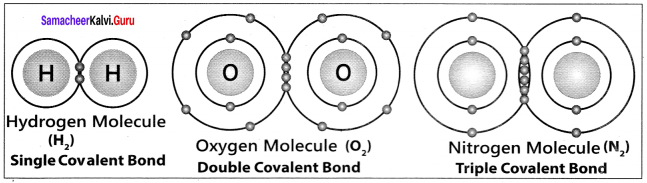
Question 3.
Explain the Postulates of VSEPR theory.
Answer:
- The shape of the molecule depends on the number of valence shell electron pair around the central atom.
- There are two types of electron pairs namely bond pairs and lone pairs. The bond pair of electrons are those shared between two atoms, while the lone pairs are the valence electron pairs that are not involved in bonding.
- Each pair of valence electrons around the central atom repels each other and hence, they are located as far away as possible in three dimensional space to minimize the repulsion between them.
- The repulsive interaction between the different types of electron pairs is in the following order.
1p – 1p > 1p – bp > bp – bp
1p – lone pair; bp – bond pair
- The lone pair of electrons are localised only on the central atom and interacts with only one nucleus whereas the bond pairs are shared between two atoms and they interact with two nuclei.
- Because of this lone pairs occupy more space and have greater repulsive power than the bond pairs in a molecule.
Question 4.
Define coordinate covalent bond. Illustrate the formation of coordinate covalent bond with a suitable example.
Answer:
1. In the bond formation, one of the combining atoms donates a pair of electrons i.e., two electrons which are necessary for the covalent bond formation and these electrons are shared by both the combining atoms, and the bond formed is called coordinate covalent bond.
2. The combining atom which donates the pair of electron is called the donor atom and the other atom is called the acceptor atom. This bond is denoted by an arrow starting from the donor atom pointing towards the acceptor atom.
3. For example, in ferricyanide ion [Fe(CN)6]4- each cyanide ion (CN–) donates a pair of electrons to form a coordinate bond with iron (Fe2+) and these electrons are shared by Fe2+ and CN– ions.
4. 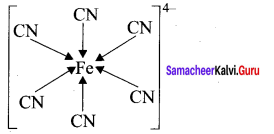
5. Ammonia having a lone pair of electrons donates its pair to an electron deficient molecule such as BF3 to form a coordinate covalent bond
Question 5.
Explain about valence bound theory for the formation of H2 molecule.
Answer:
1. Two hydrogen atoms Ha and Hb are separated by infinite distance. At this stage, there is no interaction between these two atoms and the potential energy of this system is arbitrarly taken as zero.
2. As these two atoms approach each other, in addition to electrostatic attractive forces between the nucleus and its own electrons, the following new forces begins to operate
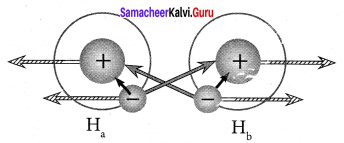
3. The new attractive forces  arise between:
arise between:
- nucleus of Ha and valence electron of Hb
- nucleus of Hb and the valence electron of Ha
4. The new repulsive forces 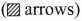 arise between:
arise between:
- the nucleus Of Ha and Hb
- the valence electrons of Ha and Hb
5. The attractive forces tend to bring Ha and Hb together whereas the repulsive forces tends to push them apart.
6. At the initial stage, as the two hydrogen atoms approach each other, the attractive forces are stronger than repulsive forces and the potential energy decreases.
7. A stage is reached where the net attractive forces are exactly balanced by repulsive forces and the potential energy of the system acquires a minimum energy.
8. At this stage, there is a maximum overlap between the atomic orbitals of Ha and Hb and atoms Ha and Hb are now said to be bonded together by a covalent bond.
Question 6.
What arc the salient features of Valence Bond (VB) theory?
Answer:
1. When half filled orbitals of two atoms overlap, a covalent bond will be formed between them.
2. The resultant overlapping orbitals are occupied by the two electrons with opposite spins. For example when H2 is formed, the two is electron of two hydrogen atoms get paired up and occupy the overlapped orbitals.
3. The strength of a covalent bond depends upon the extent of overlap of atomic orbitals. Greater the overlap, larger is the energy released and stronger will be the bond formed.
4. Each atomic orbital has a specific direction (excepts-orbital which is spherical) and hence orbital overlap takes place in the direction that maximises overlap.
5. Depending upon the nature of overlap, the bonds are classified as σ covalent bond and π it covalent bond.
6. When two atomic orbitals overlap linearly along the axis, the resultant bend is called a sigma (σ) bond. This overlap is also called or “axial overlap”.
7. When two atomic orbitals overlap sideways the resultant covalent bond is called a pi (π) bond.

Question 7.
Explain about sp hybridisation with suitable example.
Answer:
- bond rormation in Beryllium chloride takes place by sp hybridisation.
- The valence shell of Beryllium has the electronic configuration as follows:
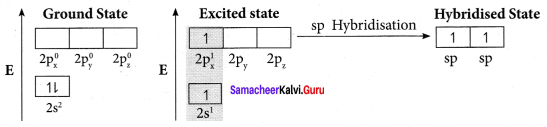
3. In BeCl2, both the Be – Cl bonds are equivalent and it was observed that the molecule is linear. VB theory explains this observed behaviour by sp hybridisation. One of the paired electrons in the 2s orbital gets excited to 2p orbital.
4. Now the 2s and 2p orbitals hybridise and produce two equivalent sp hybridised orbitals which have 50% s-character and 50% p-character. These sp hybridised orbitals are oriented in opposite direction.
5. Each of the sp hybridised orbitals linearly overlap with p orbital of the chlorine to form a covalent bond between Be and Cl atoms as follow:

Question 8.
Explain the formation of methane using VB theory?
Answer:
1. Methane is formed by sp3 hybridisation. In CH4 molecule, the central carbon atom is bounded to four hydrogen atoms.
2. The ground state valence shell electronic configuration of carbon is [He] 2s22px2 2py1 2px0
3. En order to form four covalent bonds with the four hydrogen atoms, one of the paired electrons in 2s orbital of carbon is promoted to its 2Pz orbital in the excited state.
4. The one 2s orbital and three 2p orbitals of carbon atom mixes to give four equivalent sp3 hybridised orbitals. The angle between any of the two sp3 hybridised orbitals is 109°•28’
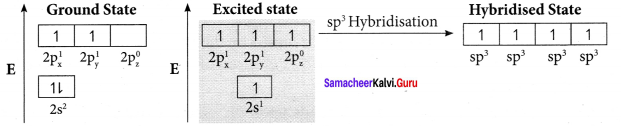
5. The Is orbital of the four hydrogen atoms overlap linearly with the four sp3 hybridised orbitais of carbon to form four C – H σ bonds in the methane molecule as follows
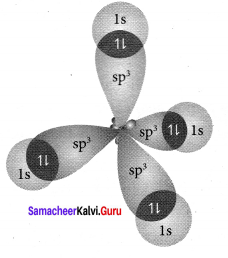
Question 9.
Explain sp3d hybridisation with a suitable example.
Answer:
1. In the PCl5 molecule, the central atom phosphorous is covalently bonded to five chlorine atoms. Here the atomic orbitals of phosphorous undergoes sp3d2 hybridisation which involves its one 3s orbital, three 3p orbitals and one vacant 3d orbital (dz2)
2. The ground state electronic configuration of phosphorous is [Ne] 3s23px23py13pz1
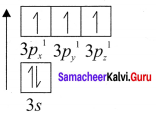
3. One of the paired electrons in the 3s orbital of phosphorous ¡s promoted to one of its vacant 3d orbital (dz2) in the excited state.
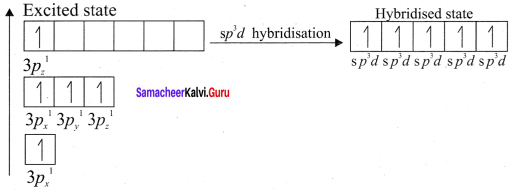
4. The 3pz orbitals of the five chlorine atoms linearly overlap along the axis with the five sp3d hybridised orbitals of phosphorous to form the five P – CI bonds as follows.
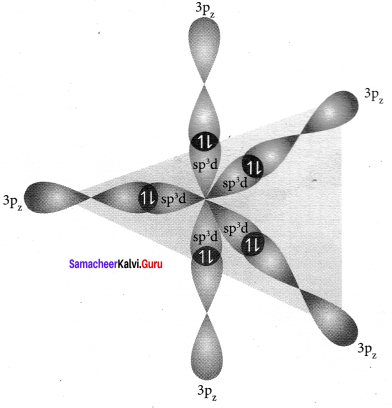
Question 10.
Explain about. sp3d2 hybridisation with an example.
Answer:
1. In sulphur hexafluoride SF6, the central atom sulphur extend its octet to undergo sp3d hybridisation to generate six sp3d2 hybridised orbitals which accounts for six equivalent S – F bonds.
2. The ground state electronic configuration of sulphur is [Ne] 3s23px13py13pz1
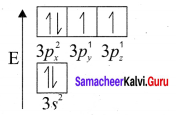
3. One electron each form 3s orbital and 3p orbital of sulphur is promoted to its two vacant 3d orbitals dz2 and dx2-y2 in the excited state.
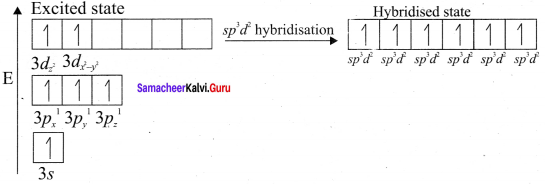
4. A total of six valence orbitals from sulphur (one 3s orbital, three 3p orbitals and two 3d orbitals) (dx2 and dx2-y2) which mixes to give six equivalent sp3d2 hybridised orbitals. The orbital geometry is octahedral.
5. The six sp3d2 hybridised orbitals of sulphur overlaps linearly with 2pz orbital of six fluorine atoms to form the six S – F bonds in sulphur hexa fluoride.
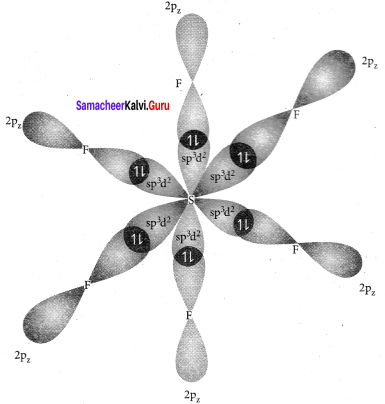
Question 11.
Explain about the salient features of molecular orbital theory.
Answer:
1. When atoms combine to form molecules, their individual atomic orbitals lose their identity and form new orbitals called molecular orbitals.
2. The shape of molecular orbitals depend upon the shapes of combining atomic orbitals.
3. The number of molecular orbitals formed is the same as the number of combining atomic orbitals. half the number of molecular orbitals formed will have lower energy and are called bonding orbitals, while the remaining half molecular orbitals will have higher energy and are called anti-bonding molecular orbitals.
4. The bonding molecular orbitals are represented as σ (sigma), π (pi), δ (delta) and the corresponding anti-bonding orbitals are called σ*, π* and δ*.
5. The electrons in the molecule are accommodated in the newly formed molecular orbitals. The filling of electrons in these orbitals follow Autbau’s Principle, Pauli’s exclusion principle and Hund’s rule as in the case of filling of electrons in the atomic orbitals.
6. Bond order gives the number of covalent bonds between the two combining atoms. The bond order of a molecule can be calculated using
the foLlowing equation:

Nb = Number of electrons in bonding molecular orbitals.
Na = Number of electrons in anti-bonding molecular orbitals.
7. A bond order of zero value indicates that the molecule does not exist.
Question 12.
Explain the MO diagram for NO molecule.
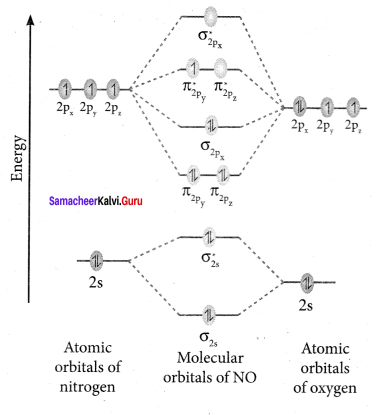
Answer:
- Electronic configuration of N atom is 1s2 2s2 2p3
- Electronic configuration of O atom is 1s2 2s2 2p4
- Electronic configuration of NO molecule is
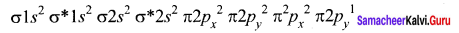
- Bond order
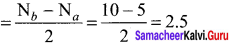
- NO molecule has one unpaired electron, hence it is paramagnetic.
Question 13.
Write the postulates of molecular orbital theory.
Answer:
- When atoms combines to form molecules, their individual atomic orbitals lose their identity and forms new orbitals called molecule orbitals.
- The shapes of molecular orbitals depend upon the shapes of combining atomic orbitals.
- The number of molecular orbitals formed is the same as the number of combining atomic orbitals. Half the number of molecular orbitals formed will have lower energy than the corresponding atomic orbital, while the remaining molecular orbitals will have higher energy.
- The molecular orbital with lower energy is called bonding molecular orbital and the one with higher energy is called an anti-bonding molecular orbital. The bonding molecular orbitals are represented as σ (Sigma), π (pi), δ (delta) and the corresponding antibonding orbitals are denoted as σ*, π* and δ*.
- The electrons in a molecule are accommodated in the newly formed molecular orbitals. The filling of electrons in these orbitals follows Aufbau’s principle, Pauli’s exclusion principle, and Hund’s rule as in the case of filling of electrons in atomic orbitals.
- Bond order gives the number of covalent bonds between the two combining atoms. The bond order of a molecule can be calculated using the following equation
- Bond order = \(\frac{\mathrm{N}_{\mathrm{b}}-\mathrm{N}_{\mathrm{a}}}{2}\)
Where, Nb = Total number of electrons present in the bonding molecular orbitals.
Na = Total number of electrons present in the antibonding molecular orbitals.
A bond order of zero value indicates that the molecule doesn’t exist.
Question 14.
Explain the bonding in metals by molecular orbital theory.
Answer:
1. According to molecular orbital theory the atomic orbitals of large number of atoms in a crystal overlap to form numerous bonding and anti-bonding molecular orbitals without any band gap.
2. The bonding molecular orbitals are completely filled with an electron pair in each and the anti-bonding molecular orbitals are empty.
3. Absence of band gap accounts for high electrical conductivity of metals.
4. High thermal conductivity is due to thermal excitation of many electrons from the valence band to the conduction band.
5. With an increase in temperature, the electrical conductivity decreases due to vigorous thermal motion of lattice ions that disrupts the uniform lattice structure. that is required for free motion of electrons within the crystal.
Common Errors
- The number of bonds formed by elements may go wrong.
- When writing Lewis structure, electrons may be written in an irregular way.
- Coordinate covalent bond should not be written as a line
Rectifications
- Always hydrogen and fluorine form 1 bond Oxygen 2 bonds, Nitrogen 3 bonds, Carbon 4 bonds
- When writing lewis structure, each atom should be surrounded by eight electrons in such a way as 4 pairs of electrons.
- Coordinate covalent bond should be written from donor atom to acceptor atom as an arrow mark Donor-Acceptor



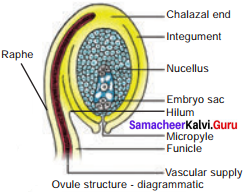
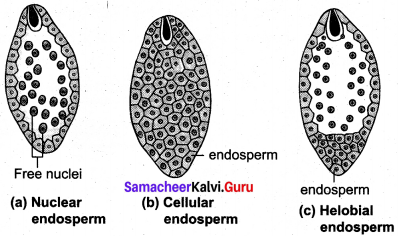



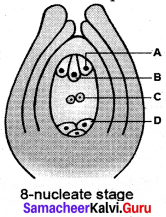

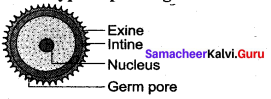
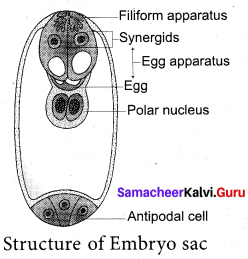
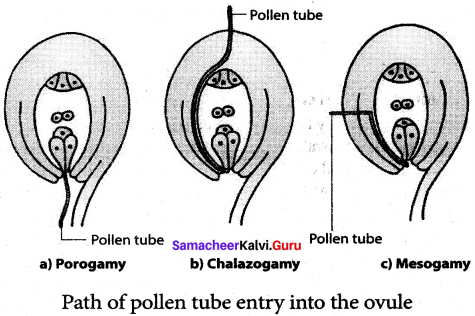
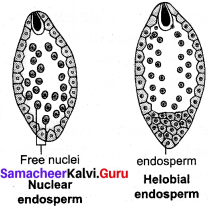
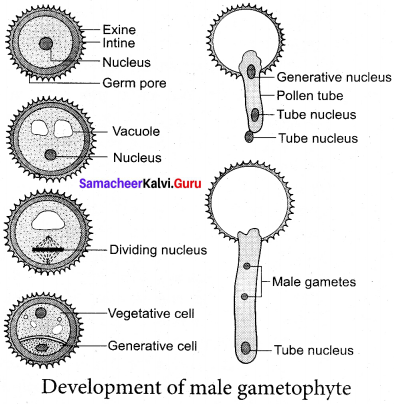
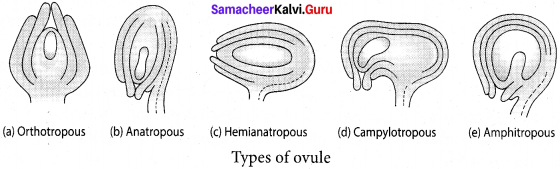
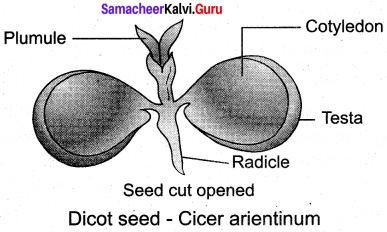

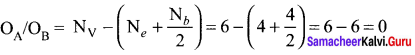










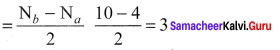


















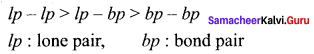







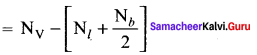
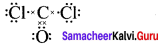
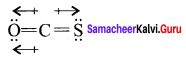




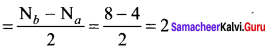




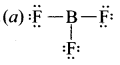












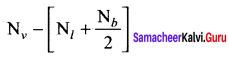
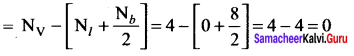
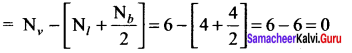
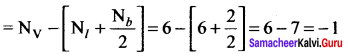
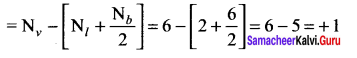

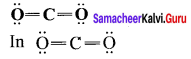
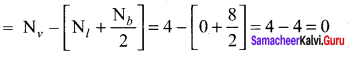
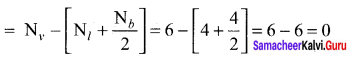
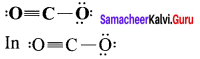
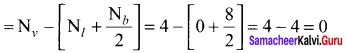
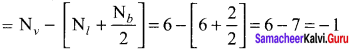
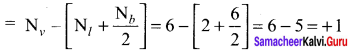
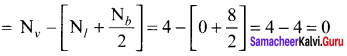
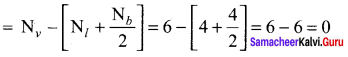
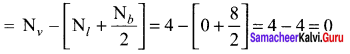
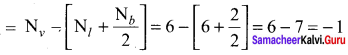
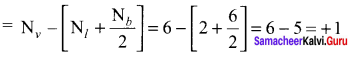
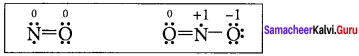









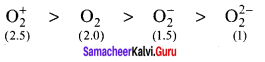


















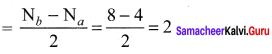

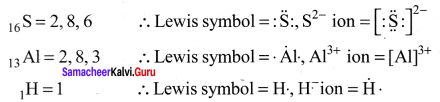



 there are two bond pairs and two lone pairs.
there are two bond pairs and two lone pairs.



















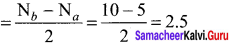
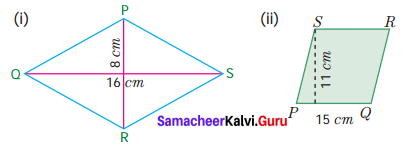
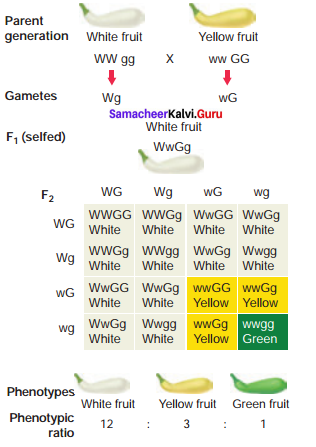
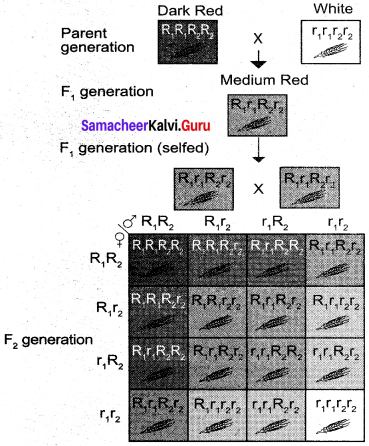
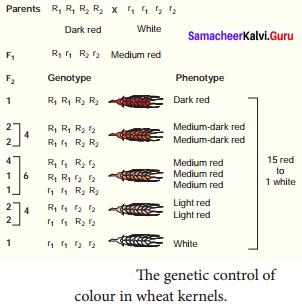
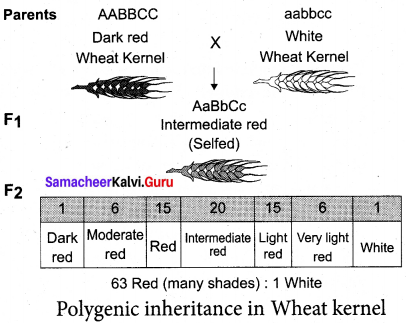
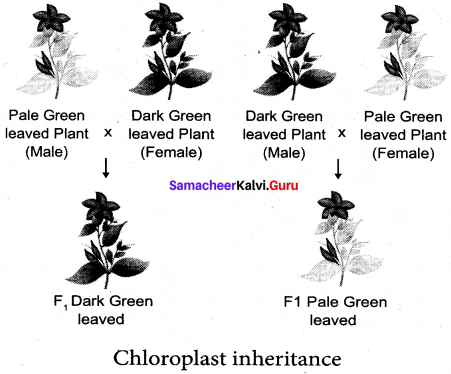


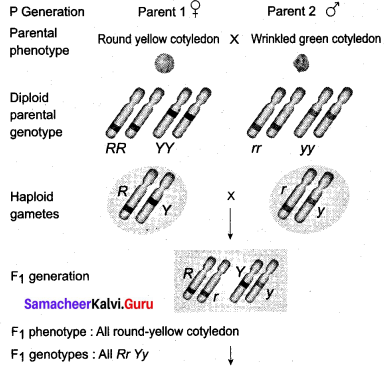
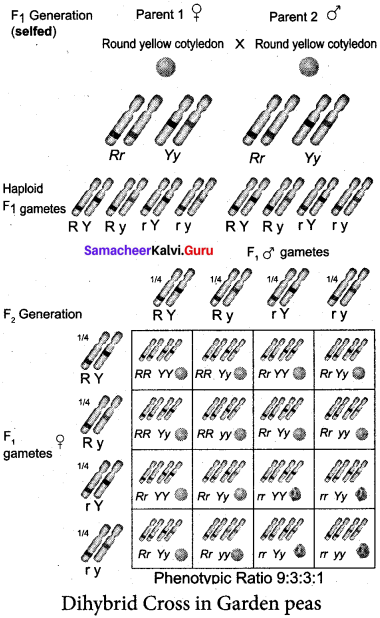
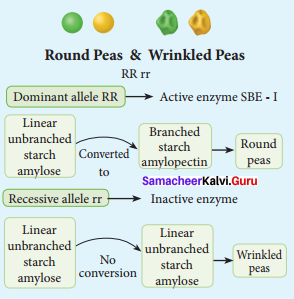
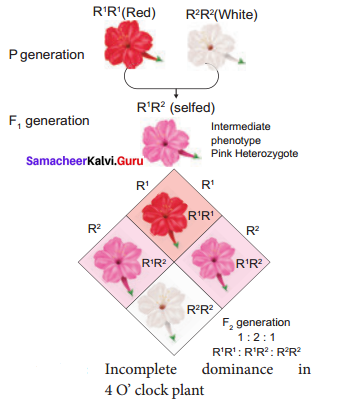
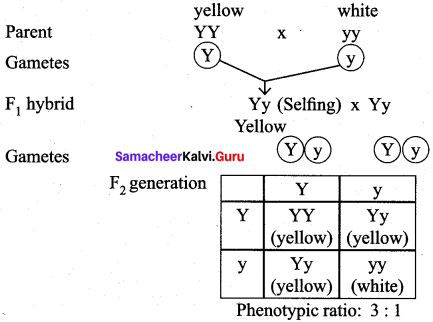
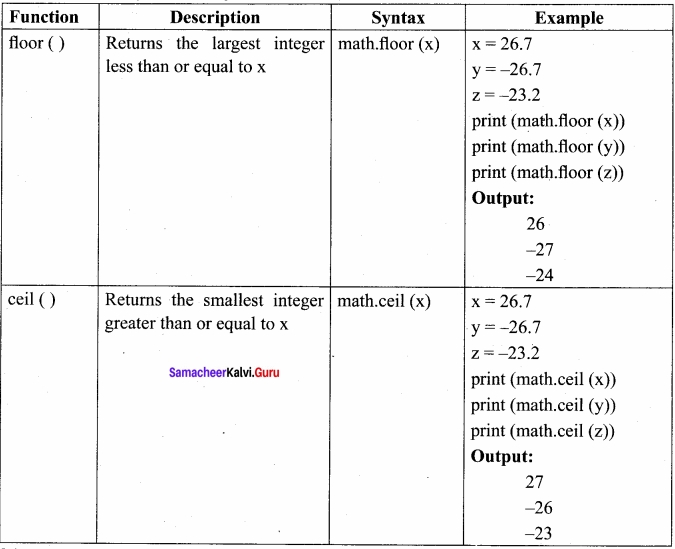
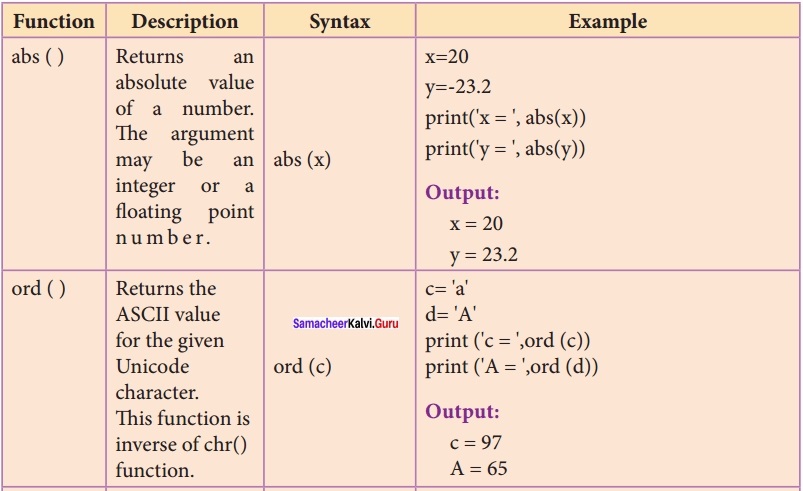
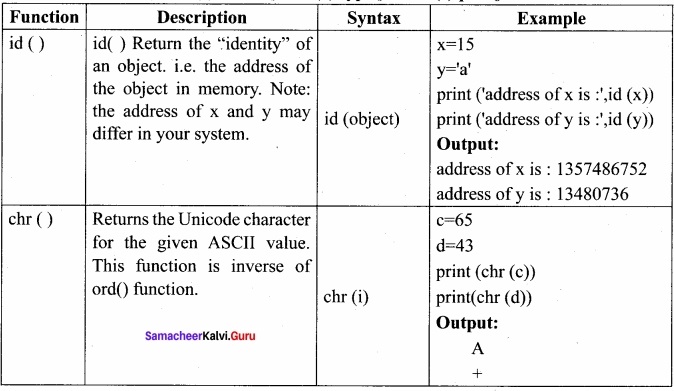
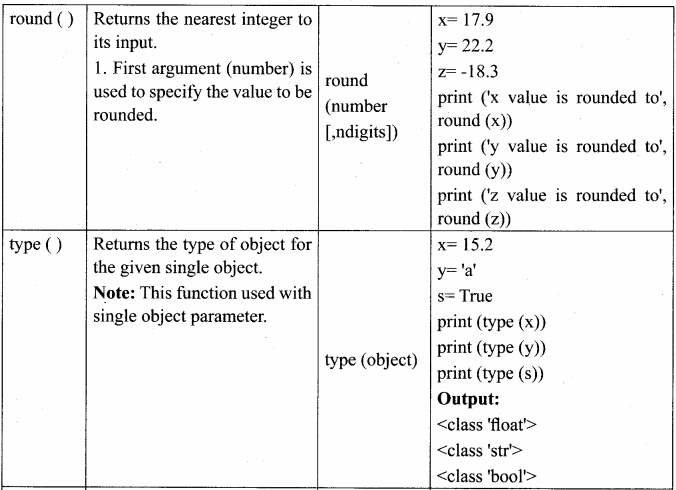
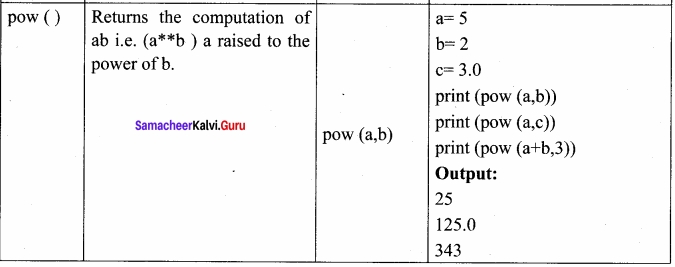
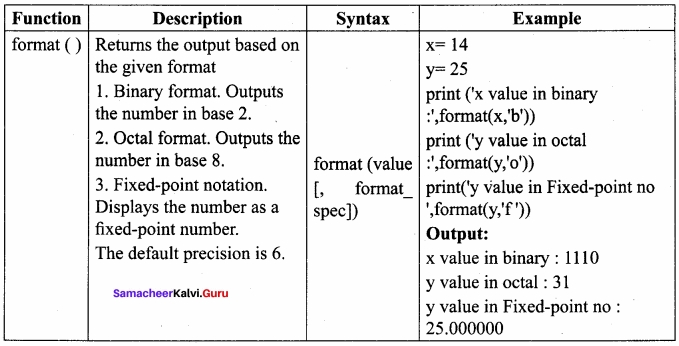
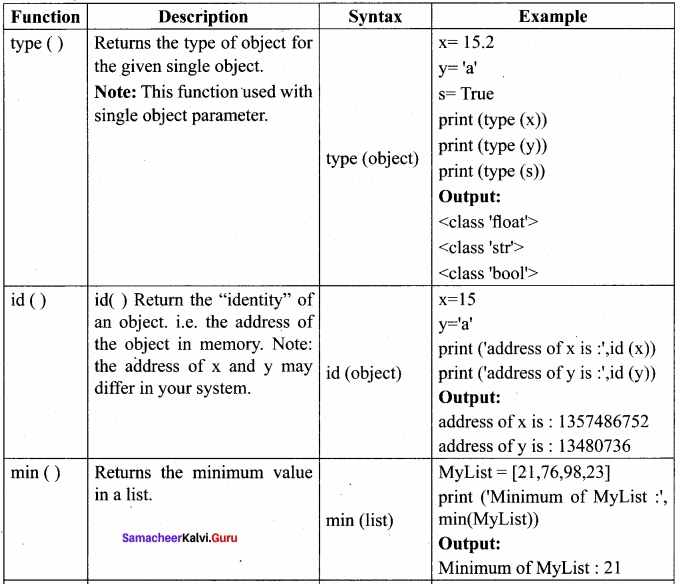
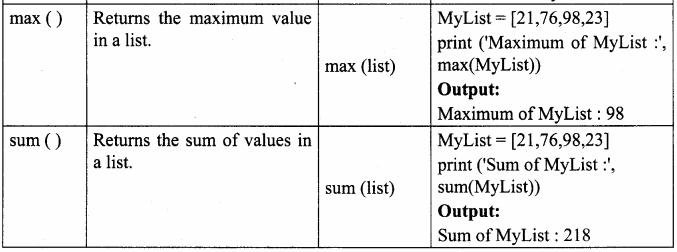


















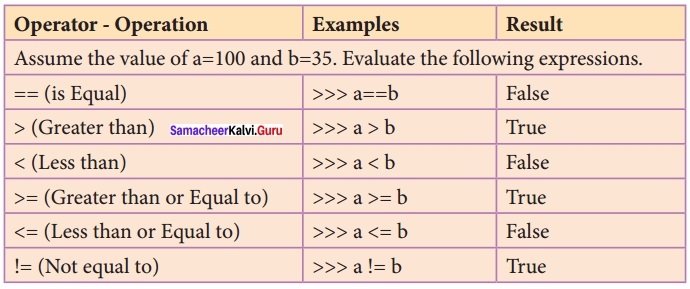






 x 100
x 100
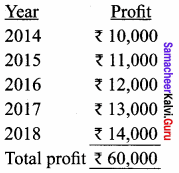
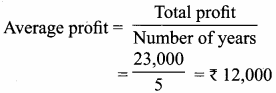
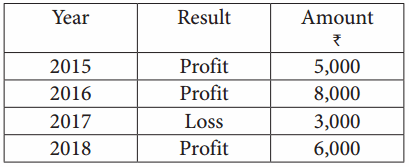
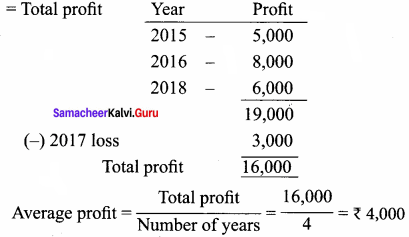
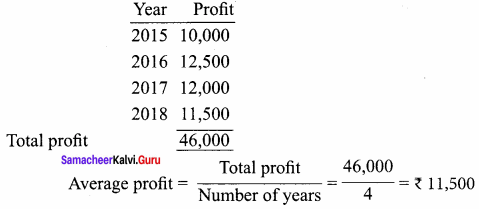
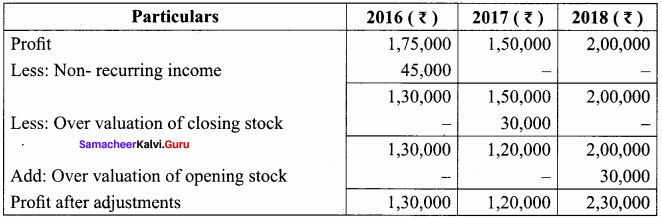
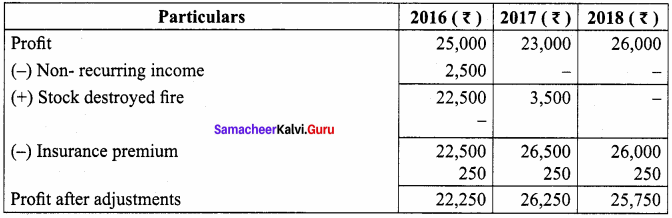
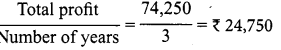
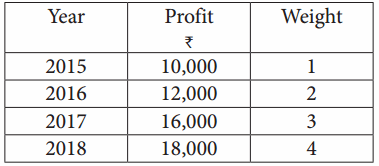
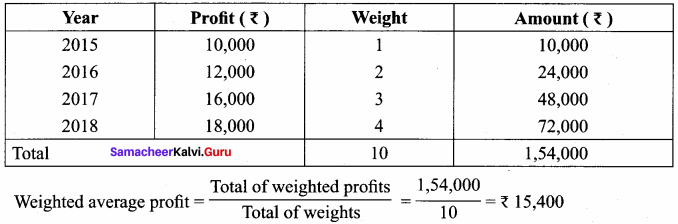
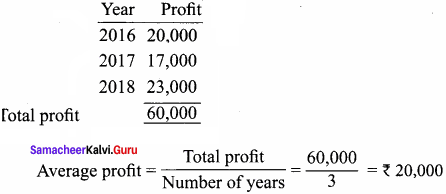
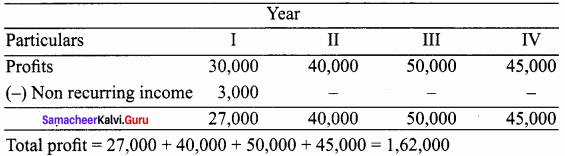
 = \(\frac { 1, 62, 000 }{ 4 }\)
= \(\frac { 1, 62, 000 }{ 4 }\)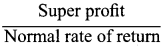 x 100
x 100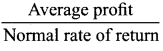 x 100
x 100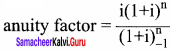
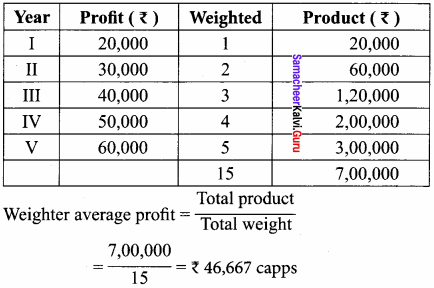

 x 100
x 100