Students can Download Chemistry Chapter 13 Hydrocarbons Questions and Answers, Notes Pdf, Samacheer Kalvi 11th Chemistry Solutions Guide Pdf helps you to revise the complete Tamilnadu State Board New Syllabus and score more marks in your examinations.
Tamilnadu Samacheer Kalvi 11th Chemistry Solutions Chapter 13 Hydrocarbons
Samacheer Kalvi 11th Chemistry Hydrocarbons Textual Evaluation Solved
Samacheer Kalvi 11th Chemistry Hydrocarbons Multiple Choice Questions.
Question 1.
The correct statement regarding the comparison of staggered and eclipsed conformations of ethane is ……….. [NEET]
(a) the eclipsed conformation of ethane is more stable than staggered conformation even though the eclipsed conformation has torsional strain.
(b) the staggered conformation of ethane is more stable than eclipsed conformation, because staggered conformation has no torsional strain.
(c) the staggered conformation of ethane is less stable than eclipsed conformation, because staggered conformation has torsional strain.
(d) the staggered conformation of ethane is less stable than eclipsed conformation, because staggered conformation has no torsional strain.
Answer:
(b) the staggered conformation of ethane is more stable than eclipsed conformation, because staggered conformation has no torsional strain.
Question 2.
![]() The above reaction is an example of which of the following?
The above reaction is an example of which of the following?
(a) Reirner Tiemann reaction
(b) Wurtz reaction
(c) Aldol condensation
(d) Hoffmann reaction
Answer:
(b) Wurtz reaction
Question 3.
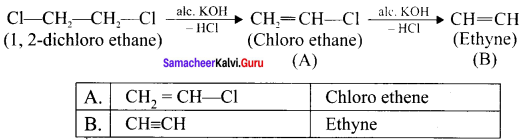
An alkyl bromide (A) reacts with sodium in ether to form 4, 5-diethyloctane, the compound (A) is ……….
(a) CH3(CH2)3Br
(b) CH3(CH2)5Br
(c) CH3(CH2)3CH(Br)CH3
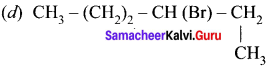
Answer:
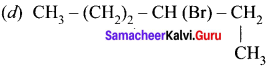
Question 4.
The C-H bond and C-C bond in ethane are formed by which of the following types of overlap ………..
(a) sp3 – s and sp3 – sp3
(b) sp2 – s and sp3 – sp3
(c) sp – sp and sp – sp
(d) p – s and p – p
Answer:
(a) sp3 – s and sp3 – sp3
Question 5.
In the following reaction 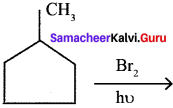
the major product obtained is ………

Answer:
Question 6.
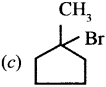
Which of the following is optically active?
(a) 2 – Methylpentane
(b) Citric acid
(c) Glycerol
(d) none of these
Answer:
(a) 2 – Methylpentane
![]()
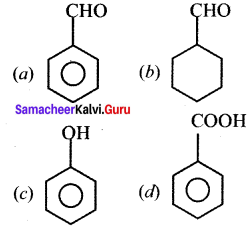
Question 7.
The compounds formed at anode in the electrolysis of an aqueous solution of potassium acetate are ……….
(a) CH4 and H2
(b) CH4 and CO2
(c) C2 H6 and CO2
(d) C2 H6 and Cl2
Answer:
(c) C2 H6 and CO2
Question 8.
The general formula for cycloalkanes is …………
(a) CnHn
(b) CnH2n
(c) CnH2n-2
(d) CnH2n+2
Answer:
(b) CnH2n
Question 9.
The compound that will react most readily with gaseous bromine has the formula ………….[NEET]
(a) C3H6
(b) C2H2
(c) C4H10
(d) C2H4
Answer:
(a) C3H6
Question 10.
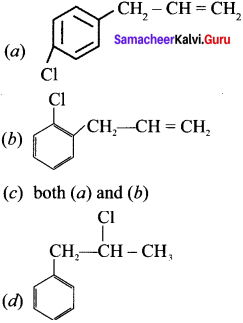
Which of the following compounds shall not produce propene by reaction with HBr followed by elimination (or) only direct elimination reaction? [NEET]
![]()
(b) CH3 – CH2 – CH2 – OH
(c) H2C – C = O
(d) CH3 – CH2 – CH2Br
Answer:
(c) H2C = C = O
Question 11.
Which among the following alkenes on reductive ozonolysis produces only propanone?
(a) 2 – Methylpropene
(b) 2 – Methylbut – 2 – ene
(c) 2, 3 – Dimethylbut – 1 – ene
(d) 2, 3 – Dimethylbut – 2 – ene
Answer:
(d) 2, 3 – Dimethylbut – 2 – ene
![]()
Question 12.
The major product formed when 2 bromo – 2 – methylbutane is refluxed with ethanolic KOH is ……..
(a) 2 – methylbut – 2 – ene
(b) 2 – methylbutan – 1 – ol
(c) 2 – methyl but – 1 – ene
(d) 2 – methylbutan -2- ol
Answer:
(a) 2 – methylbut – 2 – ene
Question 13.
Major product of the below mentioned reaction is ……….
![]()
(a) 2 – chloro – 1 – iodo – 2 – methylpropane
(b) 1 – chloro – 2 – iodo – 2 – methylpropane
(c) 1 ,2 – dichioro – 2 – methylpropane
(d) 1, 2 diiodo 2 – methylpropane
Answer:
(a) 2 – chioro – 1 – lodo – 2 – methylpropane
Question 14.
The IUPAC name of the following compound is ………
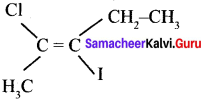
(a) trans – 2 – chloro-3 iodo – 2 – pentane
(b) cis – 3 – iodo – 4 – chloro – 3 – pentane
(c) trans – 3 – iodo – 4 – chloro – 3 – pentene
(d) cis – 2 chloro – 3 – lodo -2 – pdntene
Answer:
(a) trans -2 – chloro -3 – iodo – 2 – pentane
Question 15.
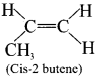
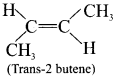
cis – 2 – butene and trans – 2 – butene are ……….
(a) conformational isomers
(b) structural isomers
(c) configurational isomers
(d) optical isomers
Answer:
(c) configurational isomers
![]()
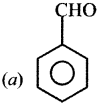
Question 16.
Identify the compound (A) in the following reaction.
Answer:
Question 17.
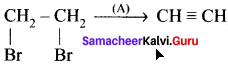 where a is ………
where a is ………
(a) Zn
(b) Conc. H2SO4
(c) Alc. KOH
(d) Dil. H2SO4
Answer:
(c) Alc. KOH
Question 18.
Consider the nitration of benzene using mixed conc. FeSO4 and HNO3, if a large quantity of KHSO4 is added to the mixture, the rate of nitration will be ………
(a) unchanged
(b) doubled
(c) faster
(d) slower
Answer:
(d) slower
Question 19.
In which of the following molecules, all atoms are co-planar?
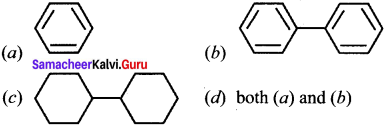
Answer:
(d) both (a) and (b)
Question 20.
Propyne on passing through red hot iron tube gives ………
Answer:
Question 21.

Answer:
Question 22.
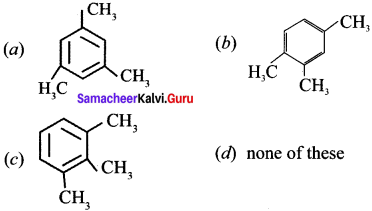
Which one of the following is non-aromatic?
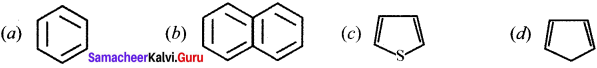
Answer:
![]()
Question 23.
Which of the following compounds will not undergo Friedal – crafts reaction easily? [NEET]
(a) Nitrobenzene
(b) Toluene
(c) Cumene
(d) Xyiene
Answer:
(a) Nitrobenzene
![]()
Question 24.
Some meta-directing substituents in aromatic substitution are given. Which one is most deactivating?
(a) – COOH
(b) – NO2
(c) – C N
(d) – SO3H
Answer:
(b) – NO2
Question 25.
Which of the following can be used as the halide component for friedal – crafts reaction?
(a) Chiorobenzene
(b) Bromobenzene
(c) Chloroethene
(d) Isopropyl chloride
Answer:
(d) Isopropyl chloride
Question 26.
An alkane is obtained by decarboxylation of sodium propionate. Same alkane can be prepared by ……..
(a) Catalytic hydrogenation of propene
(b) action of sodium metal on iodomethane
(c) reduction of 1 – chloropropane
(d) reduction of bromomethane
Answer:
(b) action of sodium metal on iodomethane
Question 27.
Which of the following is aliphatic saturated hydrocarbon?
(a) C8H18
(b) C9H18
(c) C8H14
(d) All of these
Answer:
(a) C8H18
Question 28.
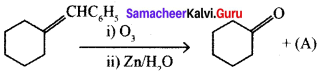
Identify the compound ‘Z’ in the following reaction.
![]()
(a) Formaldehyde
(b) Acetaldehyde
(c) Formic acid
(d) None of these
Answer:
(a) Formaldehyde
Question 29.
Peroxide effect (Kharasch effect) can be studied in case of ………
(a) Oct – 4 – ene
(b) Hex – 3 – ene
(c) Pent – 1 – ene
(d) But – 2 – ene
Answer:
(a) Pent – 1 – ene
![]()
Question 30.
2 – butyne on chlorination gives ………
(a) 1 – chlorobutane
(b) 1, 2 – dichlorobutane
(c) 1, 1, 2, 2 – tetrachlorobutane
(d) 2, 2, 3, 3 – tetrachlorobutane
Answer:
(d) 2, 2, 3, 3 tetra chiorobutane
Samacheer Kalvi 11th Chemistry Hydrocarbons Short Answer Questions
Question 31.
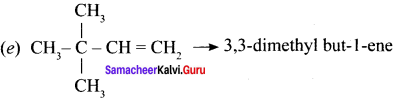
Give IUPAC names for the fllowing compounds …………
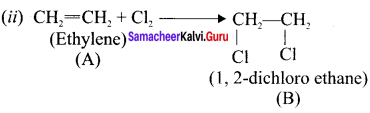
(i) CH3 CH = CH – CH= CH – C ≡C – CH3
(ii) 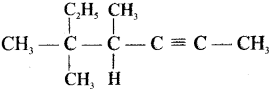
(iii) (CH3)3 C-C≡C-CH (CH3)2
(iv) Ethyl – isopropyl – acetytene
(v) CH≡C – C = C – C≡CH
Answer:
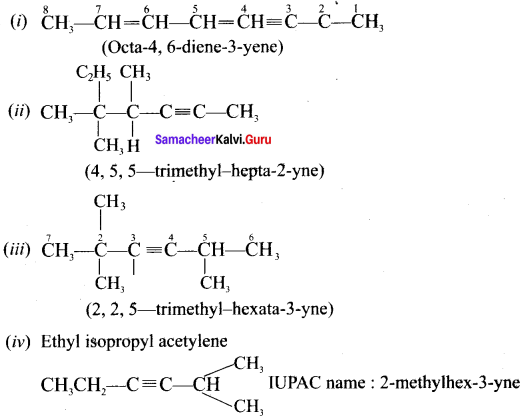
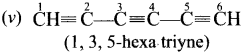
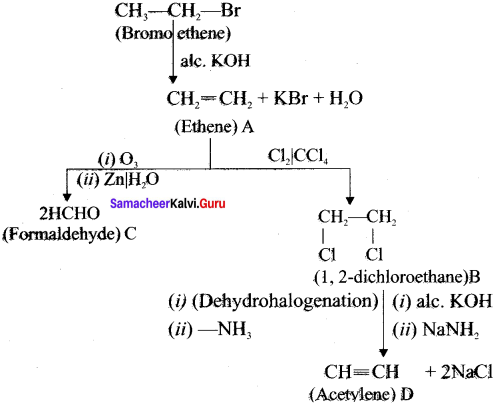
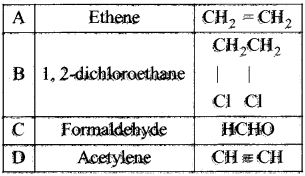
Question 32.
Identify the compound A. B, C and D in the following series of reactions.
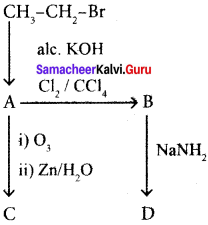
Answer:
Question 33.
Write a short note on ortho – para directors aromat&c e1ropiJic substitution reactions.
Answer:
The group which increases the eleiron deity at oìo and para positions of the ring are known as ortho-para directors.
Example:
-OH, -NH2 -NHR -CH3, -OCH3 etc.
Let us consider the directive influences of phenolic( -OH) group. Phenol is the resonace hybrid of following structure.
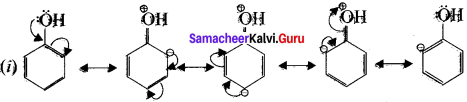
In these resonance structures the negative charge residue is present on ortho and para posrtions of the ring structure. Therefore the electron density at ortho and para positions increases as compared to the metci position, thus phenolic group activities the benzene ring for electrophilic attack at ortho and para positions and hetice – OH group is an ortho-para direction or and an activator.
![]()
Question 34.
How is propyne prepared from an alkyene dihalide?
Answer:
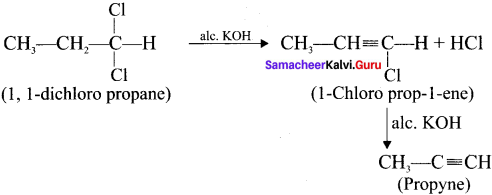
Question 35.
An alkyl halide with molecular formula C6H13Br on dehydrohalogenation gave two isomeric alkenes X and Y with molecular formula C6H12. On reductive ozonolysis, X and Y gave four compounds CH3COCH3. CH3CHO, CH3CH2CHO and (CH3)2 CHCHO. Find the alkyl halide.
Answer:
1. C6H13Br is 3 – Bromo – 4 methylpentanc.
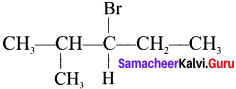
2. 3 – Bromo -4 methylpentane on dehydrogenation give two isomers X and Y as follows:
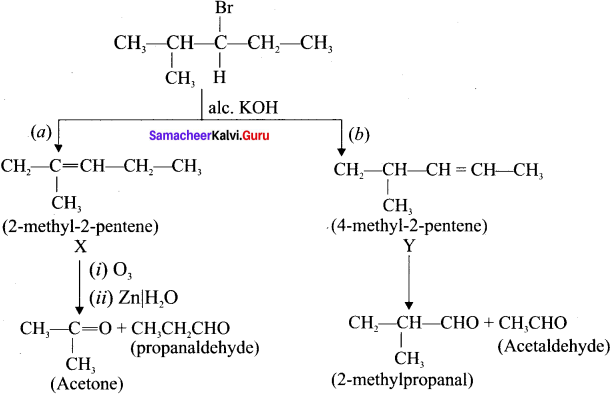
There fore C6H13 Br is 3 – Bromo – 4 – methy ipentane.
Question 36.
Describe the mechanism of Nitration of benzene.
Answer:
Step-1 :
Generation of \(\overset { \oplus }{ { NO }_{ 2 } } \) electrophile.
HNO3 + H2SO4 → \(\overset { \oplus }{ { NO }_{ 2 } } \) + H\(\overset { \oplus }{ { SO }_{ 4 } } \) + H2O
Step-2:
Attack of the electrophile on benzene ring to form arenium ion.
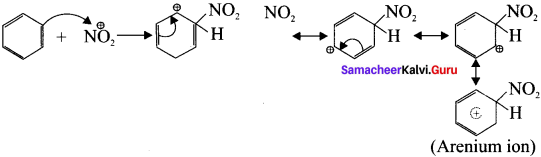
Step-3:
Rearomatisation of arenium ion.
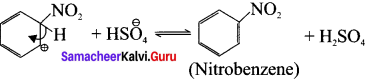
Overall Reaction:

Question 37.
How does Huckel rule help to decide the aromatic character of a compound?
Answer:
A compound is said to be aromatic, if it obeys the following rules:
- The molecule must be cyclic.
- The molecule must be co-planar.
- Complete delocalisation of it-electrons in the ring.
- Presence of (4n + 2) π electrons in the ring where n is an integer (n = 0,1,2 …)
This is known as Huckel’s rule.
Example:
1. It is cyclic one.
2. It is a co-planar molecule.
3. It has six delocalised ir electrons.
4. 4n + 2 = 6
4n = 6 – 2
4n = 4
n = 1
It obey Huckel’s rule, with n = 1, hence benzene is aromatic in nature.
![]()
Question 38.
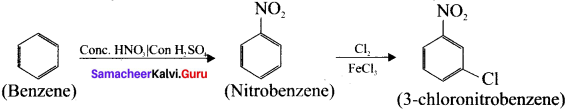
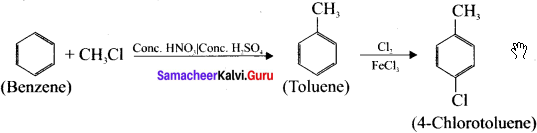
Suggest the route for the preparation of the following from benzene.
1. 3 – chioro – nitrobenzene
2. 4- chlorotoluene
3. Bromobenzene
4. in – dinitrobenzene
Answer:
1. Preparation of 3 – chloronitro – benzene from benzene:
Benzene undergoes nitration and followed by chlorination and it leads to the formation of 3- chloronitrobenzene.
2. Preparation 4-chiorotoluene from benzene:
Benzene undergoes Fnedel crafi’s alkylation followed by chlorination and it leads to the formation of 4-chiorotoluene.
3. Prepar2tion of Bromobenzene from benzene:
Bezene undergo bromination to give bromobenzene.
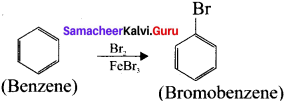
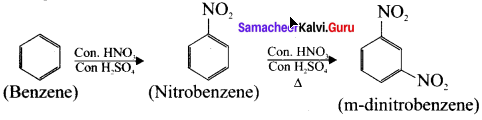
4. Preparation of m-dinitrobenzene from benzene:
Benzene undergo twice the time nitration to give m-dinitrobenzene.
Question 39.
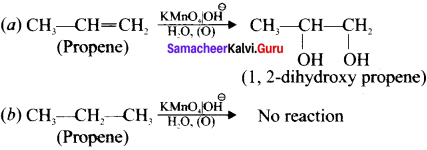
Suggest a simple chemical test to distinguish propane and propene.
Answer:
Chemical test to distinguish between propane and propene:
1. Bromine water test:
Propene contains double bond, therefore when we pour the bromine water to propene sample, it decolounses the bromine water whereas propane which is a saturated hydrocarbon does not decolourise the bromine water.
2. Baeyer’s test:
When propene reacts with Bayer’s reagent it gives 1,2 dihydroxypropene. Propane does not react with Baeyer’s reagent.
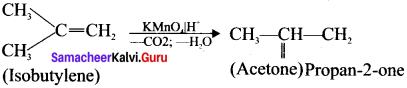
Question 40.
What happens when isobutylene is treated with acidified potassium permanganate?
Answer:
Isobutylene is treated with acidified KMnO4 to give acetone.
Question 41.
how will you convert ethyl chloride in to –
1. ethane
2. n – butane
Answer:
1. Conversion of ethyl chloride into ethane:
![]()
2. Conversion of ethyl chloride into n-butane:
Wurtz reaction:
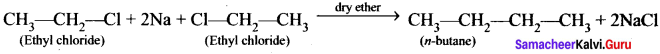
Question 42.
Describe the conformers of n-butane.
Answer:
n-butane may be considered as a derivative of ethane as one hydrogen on each carbon atom is replaced by a methyl group.
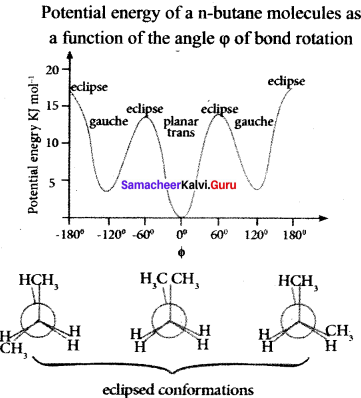
Edipsed conformation:
In this conformation, the distance between the two methyl groups is minimum so there is maximum repulsion between them and it is the least stable conformer.
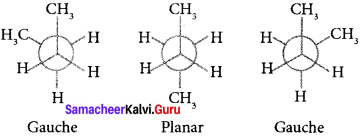
Anti or staggered form:
In this conformation, the distance between the two methyl groups is maximum and so there is minimum repulsion between them. It is the most stable conformer. The following potentially energy diagram shows the relative stability of various conformers of n-butane.
Question 43.
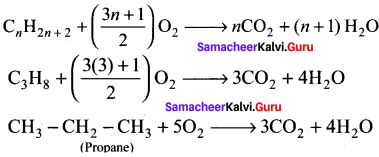
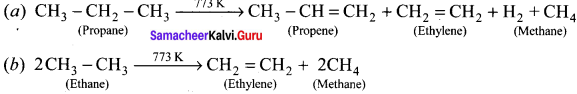
Write the chemical equations for combustion of propane.
Answer:
Chemical equations for combustion of propane:
The general combustion reaction for any alkane is:
Question 44.
Explain Markovnikoffs rule with suitable example.
Answer:
Markovnikoff’s rule: When an unsymmetrical alkene reacts with hydrogen halide, the hydrogen adds to the carbon atom that has more number of hydrogen and halogen adds to the carbon atom having fewer hydrogen atoms.
Example:
Addition of HBr to Propene:
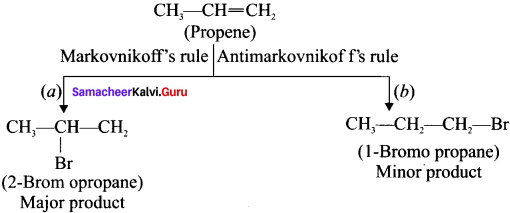
Question 45.
What happens when ethylene is passed through cold dilute alkaline potassium permanganate.
Answer:
Ethylene reacts with cold dilute alkaline KMnO4 solution to give ethylene glycol:
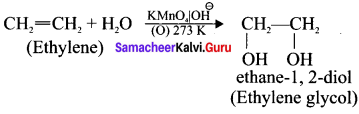
Question 46.
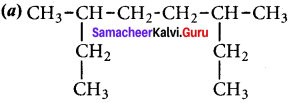
Write the structures of following alkanes.
1. 2, 3 – Dimethyl – 6 – (2 – methylpropyl) decane
2. 5 – (2 – Ethylbutyl) – 3, 3 – dimethyldecane
3. 5 (1,2 – Dimethyipropyl) – 2 – methylnonane
Answer:
1. 2, 3 – Dimethyl – 6 – (2 – rnethylpropyl) decane
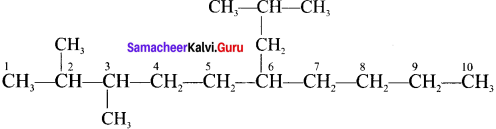
2. 5 – (2 – Ethylbutyl) – 3, 3- dimethyldecane
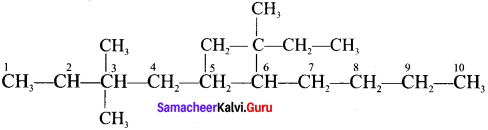
3. 5 – (1,2 – Dimethylpropyl) – 2- methylnonane
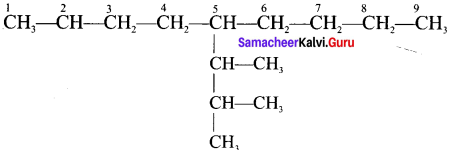
Question 47.
How will you prepare propane from a sodium salt of fatty acid?
Answer:
![]()
Sodium salt of butyric acid on heating with sodalime gives propane.
Question 48.
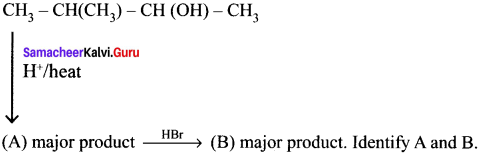
Answer:
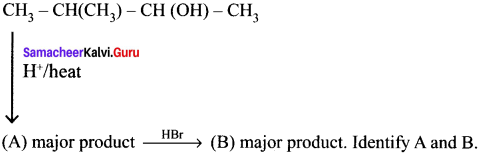
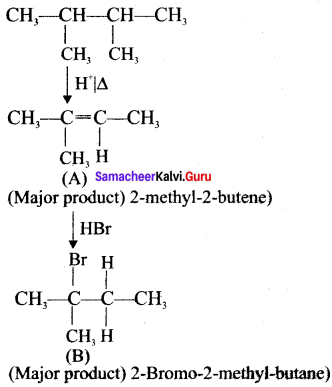
Question 49.
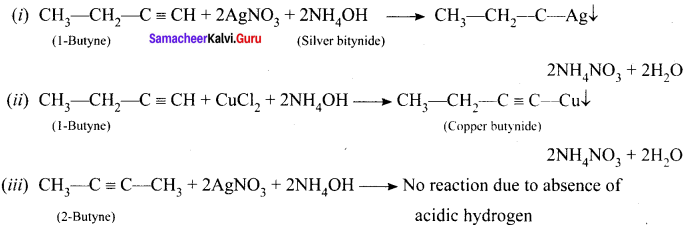
How will you distinguish 1 – butyne and 2 – butyne?
Answer:
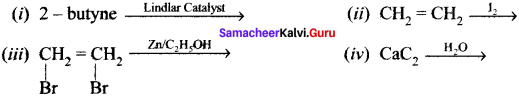
Answer:
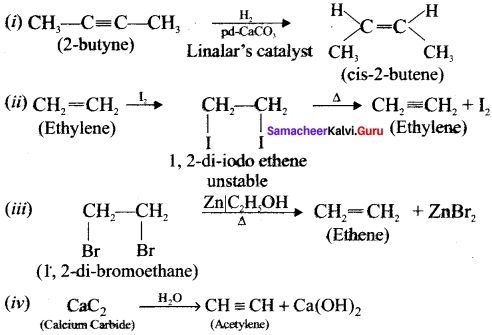
Question 50.
How will you distinguish 1 – butyene and 2 – butyne?
Answer:
![]()
In 1-butyne, terminal carbon atom contains atom one acidic hydrogen, therefore it will react with silver nitrate in the presence of ammonium hydroxide to give silver butynide. Whereas 2-butyne does not undergo such type of the reaction, because of the absence of acidic hydrogen.

![]()
Samacheer Kalvi 11th Chemistry Hydrocarbons Evaluate Your self
Question 1.
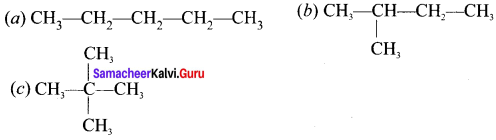
Write the structural formula and carbon skeleton formula for all possible chain isomers of C6H14 (Hexane).
Answer:
C6H14 has five possible isomeric structures:
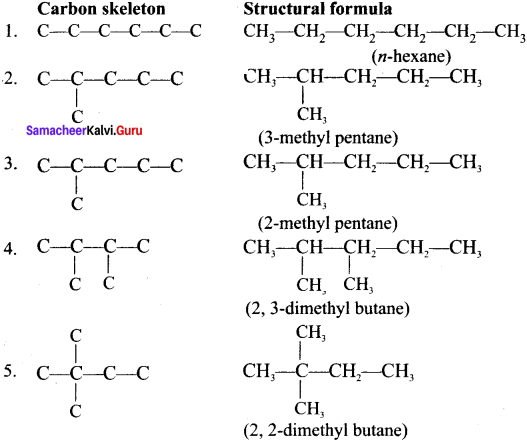
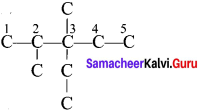
Question 2.
Give the IUPAC name for the following alkane.
Answer:
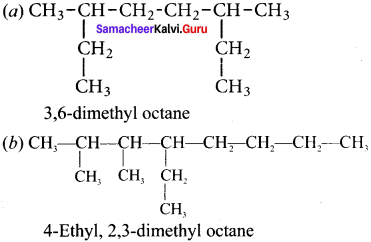
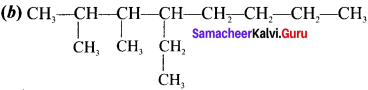
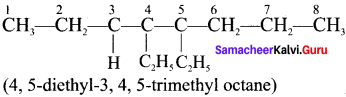
Question 3.
Draw the structural formula for 4,5 -diethyl -3,4,5-trimethyloiane
Answer:
Question 4.
Water destroys Grignard reagents. Why?
Answer:
CH3MgX + HOH → CH4 – Mg(OH)X.
Water would protonate the grignard reagent and destroy the gngnard reagent, because the grignard carbon atom is highly nucleophilic. This would form a hydrocarbon. Therefore to make a grignard solution, only ether is the best solvent and water or alcohol are not used for that purpose.
![]()
Question 5.
Is it possible to prepare methane by Kolbe’s electrolytic method.
Answer:
Kolbe’s electrolytic method is suitable for the preparation of symmetrical alkanes, that is alkanes containing even number of carbon atoms. Methane has only one carbon, hence it cannot be prepared by Kolbe’s electrolytic method.
Question 6.
Write down the combustion reaction of propane whose AH° = -2220 kJ
Answer:
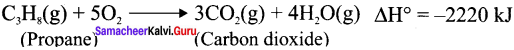
Question 7.
Why ethane is produced in chlorination of methane?
Answer:
Chlorination of methane involves free radical mechanism. During the propagation step methyl free radical is produced, which is involved in the termination step, the two methyl free radical then combines to form ethane.

Question 8.
How toluene can be prepared by this method?
(i) From n-heptane,
(ii) From 2-methyihexane
Answer:
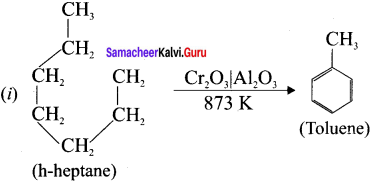
(ii)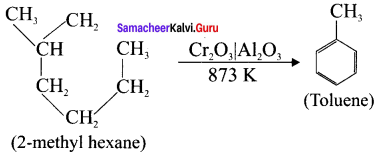
Question 9.
Write the IUPAC names for the following alkenes.
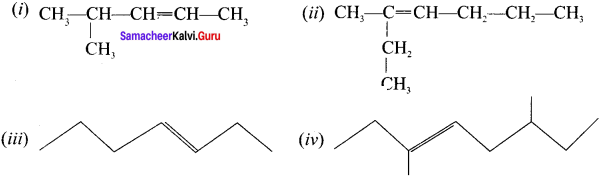
Answer:
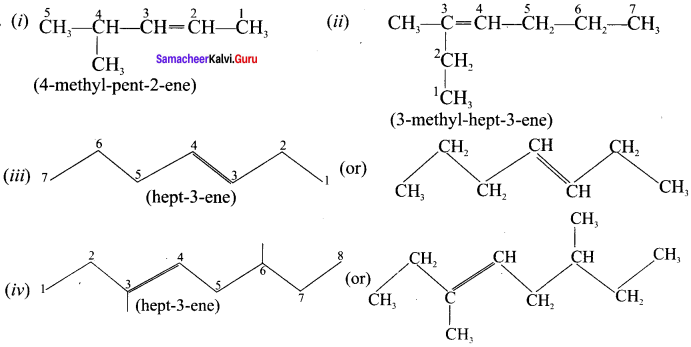
Question 10.
Draw the structures for the following alkenes.
- 6 – Bromo – 2,3 – dimethyl 2 – hexene
- 5 – Bromo – 4 – chloro 1 – heptene
- 2,5 – Dimethyl 4 – octene
- 4 – Methyl – 2 pentene
Answer:
1. 6 – Bromo – 2,3 – dirnethyl – 2 – hexene:
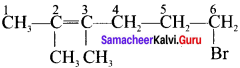
2. 5 Bromo -4 – Chloro – 1 – heptene:
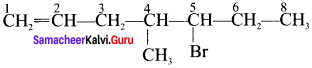
3. 2.5 – dimethyl – 4 – Octene:
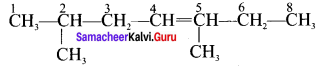
4. 4 – methyl 2 pentene:
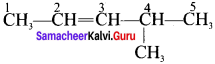
Question 11.
Draw the structure and write down the IUPAC name for the isomerism exhibited by the molecular formulae:
1. C5H10 – Pentene (3 isomers)
2. C6H12 – Hexene (5 isomers)
Answer:
1. C5H10 – Pentene (3 isomers)
(a) CH3 – CH2 – CH2 – CH = CH2 → 1- penlene (or) pent- 1-ene
(b) CH3 – CH2 – CH = CH – CH3 → 2- pentene (or) pent-2-ene

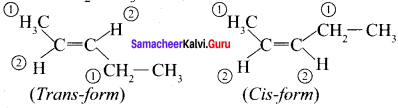
2. C6H12 – Hexene (5 isomers)
(a) CH3 – CH2 – CH2 – CH2 – CH = CH2 → Hex-I-ene
(b) CH3 – CH2 – CH2 – CH = CH – CH3 → Hex – 2 – ene
(c) CH3 – CH2 – CH = CH – CH2 – CH3 → Hex – 3 – ene
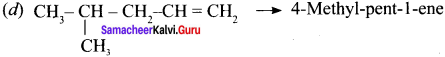
These two compounds exhibits constitutional isomerism.
Question 12.
Determine whether each of the following alkenes can exist as cis-trans isomers?
(a) 1 – Chloropropene
(b) 2 – Chloropropene
Answer:
(a) 1 – Chloropropene:
CH3 – CH = CHCl
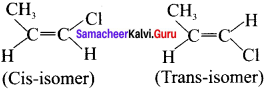
Therefore – 1 – Chloropropene has cis-trans isomers.
(b) 2 – Chloropropene:

In 2-Chloropropene, it deviates from the rule, that is “At least one same group is present on the two doubly bonded carbon atom”. Therefore-2-chloropropenc cannot exist as cis – trans isomers.
Question 13.
Draw cis-trans isomers for the following compounds
(a) 2- chloro-2-butene
(b) CH3 – CH= CH – CH2 – CH3
Answer:
(a) 2-Chloro-2-butene:

(b) CH3 – CH = CH – CH – CH3
Question 14.
How propene is prepared form 1, 2-dichloropropane?
Answer:
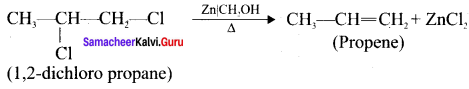
Question 15.
How ozone reacts with 2-methyl propene?
Answer:
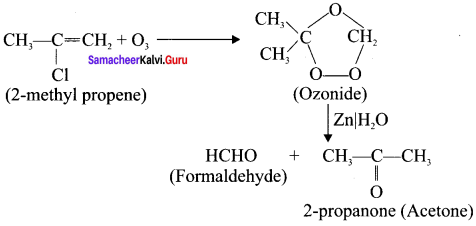
Question 16.
An organic compound (A) on ozonolysis gives only acetaldehyde. (A) reacts with Br2 /CCl4 to give compound (B). Identify the compounds (A) and (B). Write the IUPAC name of (A) and (B). Give the geometrical isomers of (A).
Answer:
2-Rutene undergo ozonolysis to give acetaldehyde only.
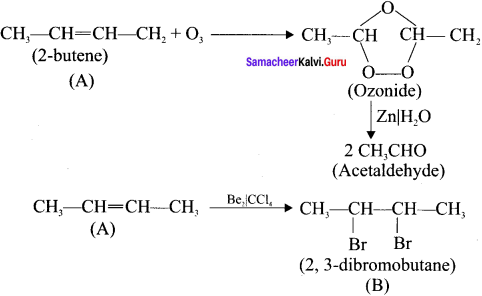
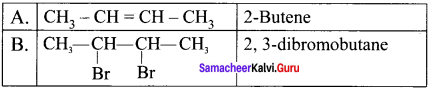
Geometrical isomers of 2 – Butene (A):

Question 17.
An organic compund (A) C2H4 decolourises bromine water. (A) on reaction with chlorine gives (Br). A reacts with HBr to give (C). Identify (A),(B),(C). Explain the reactions.
Answer:
(i) C2H4 (A), decolourises the bromine water. Therefore it contains double bond. Hence (A) is ethylene.
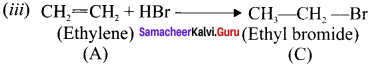
Question 18.
Prepare propyne from its corresponding alkene.
Answer:
Preparation of propyne from propene:
Question 19.
Write the products A & B for the following reaction.
Answer:
Question 20.
![]()
Answer:
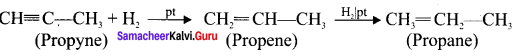
Question 21.
Calculate the number of rings present in C18H12.
Answer:
Double bond equivalent formula = ( C – \(\frac {1}{2}\) + \(\frac {1}{2}\))
Where C = no. of carbon atoms. H = no. of hydrogen and halogen atoms and N = no. of nitrogen atoms.
So. in C18H12 Double bond equkalent = 18 – \(\frac {12}{2}\) + 0 + 1 = 18 – 6 + 1 = 13
One ring is equal to one double bond equivalent.
∴ here four rings are there, four double bond equivalent arc used. So remaining, 13 – 4 = 9.
nine double bonds are present in the ring. Hence, C18H12 contain four aromatic rings.
![]()
Question 22.
write all possible isomers for an aromatic benzenoid compound having the molecular formula C8H10.
Answer:
Possible isomers for C8H10:
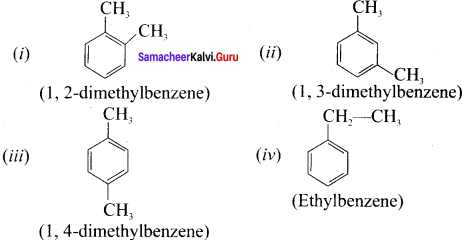
Question 23.
Write all possible isomers Iòr a monosubstituted aromatic benzenoid compound having the molecular formula C9H12
Answer:
Possible isomers for C9H12:
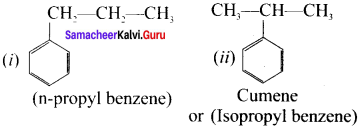
Question 24.
how benzene can be prepared by Grignard Reagent?
Answer:
Phenyl magnesium bromide reagent reacts with a water molecule to give benzene:
![]()
Question 25.
Why benzene undergoes an electrophilic substitution reaction whereas alkenes undergo an addition reaction?
Answer:
- Benzene possesses an unhybridised p-orbital containing one electron. The lateral overlap of their p-orbitals produces 3 it bond.
- The six electron of the p-orbitais cover all the six carbon atoms and arc said to be delocalised.
- Due to delocalisation, strong it-bond is formed which makes the molecule stable. Therefore benzene undergoes electrophilic substitution reaction, whereas alkenes undergo addition reaction.
Question 26.
Convert Ethyne to Benzene and name the process.
Answer:
Conversion of Ethyne into Benzene:

This process is one of the cyclic polymerisation process.
Question 27.
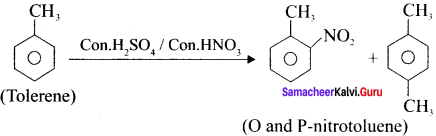
Toluene undergoes nitration easily than benzene.Why?
Answer:
1. Toluene has a methyl group on the benzene ring which is electron releasing group and hence activate the benzene ring by pushing the electrons on the benzene ring.
2. CH3 group is ortho – para director and ring activator. Therefore in toluene, ortho and para positions are the most reactive towards an electrophile, thus promoting electrophilic substitution reaction.
3. The methyl group hence makes it around 25 times more reactive than benzene. Therefore it undergoes nitration easily than benzene.
Samacheer Kalvi 11th Chemistry Hydrocarbons Additional Questions Solved
Choose the correct statement.
Question 1.
The IUPAC name of neopentane is
a) 2 – methyl butane
b) 2, 2 – dimethyl propane
c) 2 – methyl propane
d) 2, 2- dimethyl butane
Answer:
b) 2, 2 – dimethyl propane
Question 2.
Statement – I : n-butane and iso – butane are isomers.
Statement – II : Because they are having same molecular formula but differs only in the structural formula.
(a) Statement -I and II are correct and statement – II is correct explanation of statement – I
(h) Statement – I and II are correct but statement – II is not correct explanation of statement -I
(c) Statement – I is correct but statement – II s wrong.
(d) Statement – I is wrong but statement – II is correct.
Answer:
(a) Statement -I and II are correct and statement – II is correct explanation of statement-I
Question 3.
The compressed gas available in cooking gas cylinders is a mixture of:
a) C6H6 + C6H5CH3
b) C2H4 + C2H2
c) C2H4 + CH4
d) C4H10 + C3H8
Answer:
d) C4H10 + C3H8
Question 4.
Find out the branched hydrocarbon from the following compounds.
(a) 1 – propane
(b) n – propane
(c) iso – butane
(d) n – butane –
Answer:
(c) iso – butane
Question 5.
Adam’s catalyst is:
a) platinum metal
b) palladium
c) nickel metal
d) PtO2
Answer:
d) PtO2
![]()
Question 6.
Statement – I : Boiling point of methane is lower than that of butane.
Statement – II : The boiling point of continuous chain alkanes increases with increase in length of carbon chain.
(a) Statement – I and II are correct and statement – II is correct explanation of statement – I
(b) Statement – I and II are correct but statement – II is not correct explanation of statement -I
(c) Statement – I is correct but a statement – II is wrong.
(d) Statement – I is wrong but a statement – I is correct.
Answer:
(a) Statement – I and II are correct and statement – II is correct explanation of statement -I.
Question 7.
Methyl bromide is converted into ethane by heating it in an ether medium with
a) Al
b) Mg
c) Na
d) Cu
Answer:
c) Na
Question 8.
Consider the following statements.
(i) The process of reduction using sodium in liquid ammonia is called Birch reduction.
(ii) Birch reduction is stereospecific in reaction.
(iii) Aleynes can be reduced to cis – alkenes using Birch reduction. Which of the above statement is/are correct’?
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (iii) only
(d) (ii) only
Answer:
(a) (i) and (ii)
![]()
Question 9.
Statement – I : Alkenes are more reactive than alkanes.
Statement – II : Because of the presence of a double bond.
(a) Statement – I and II are correct and statement – II is correct explanation of statement – I
(b) Statement – I and Il are correct but statement – II is not correct explanation of statement -I
(c) Statement – I is correct but statement – II is wrong.
(d) Statement – I is wrong but statement – II is correct.
Answer:
(a) Statement – I and II are correct and statement – II is correct explanation of statement – I
Question 10.
Select the correct statement about alkanes
a) they are polar in nature
b) they are soluble in water
c) they are non – combustible
d) their dipole moment is zero
Answer:
d) their dipole moment is zero
Question 11.
Which one of the following has garlie odour?
(a) Ethane
(b) Ethene
(c) Ethyne
(d) Ethanol
Answer:
(c) Ethyne
Question 12.
![]() Identify the X.
Identify the X.
(a) Propane
(b) Acetone
(c) Acetaldehyde
(d) Formaldehyde
Answer:
(b) Acetone
Question 13.
Which one of the following is not a monocyclic aromatic hydrocarbon?
(a) Benzene
(b) Phenol
(c) Toluene
(d) Naphthalene
Answer:
(d) Naphthalene
Question 14.
Which one of the following is a polynuclear aromatic hydrocarbon?
(a) Anthracene
(b) Phenol
(c) Benzene
(d) Toluene
Answer:
(a) Anthracene
![]()
Question 15.
Which one of the following is an aromatic compounds?

Answer:
Question 16.
Conformational isomers are due to
a) Free rotation about C – C single bond
b) Frozen rotation about C – C single bond
e) Frozen rotation about C – C double bond
d) Restricted rotation about C – C single bond
Answer:
a) Free rotation about C – C single bond
Question 17.
Statements-I: Unlike alkenes and alkynes benzene undergoes substitution reactions rather than addition reactions under normal conditions.
Statements-II: Because of the delocalisation of electrons a strong t bond is formed which makes the molecule stable.
(a) Statement-I and II are correct and statement-II is the correct explanation of statement-I.
(b) Statement-I and II are correct but statement-II is not the correct explanation of statement-I
(c) Statement-I ¡s correct but statement-II is wrong.
(d) Statement-I is wrong but statement-II is correct.
Answer:
(a) Statement-I and II are correct and statement-II is the correct explanation of statemen-I.
Question 18.
![]() A → B + H2O. Identify A and B.
A → B + H2O. Identify A and B.
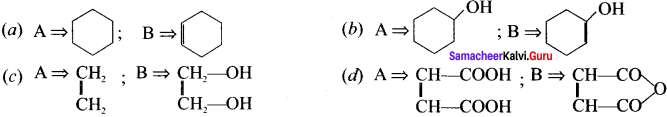
Answer:
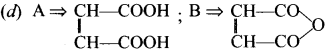
Question 19.
Benzene undergoes Birch reduction to form .

Answer:
![]()
Question 20.
How many types of carbon atoms are present in 2, 2, 3 – trimethyl pentane
a) one
b) Two
c) Three
d) Four
Answer:
d) Four
![]()
Question 21.
Which one of the following is not a meta director’?
(a) – NH2
(b) – NO2
(c) – COOR
(d) – SO3H
Answer:
(a) – NH2
Question 22.
Which one of the following benzene ring deactivator?
(a) – CHO
(b) – OH
(c) – CH3
(d) – OCH3
Answer:
(a) – CHO
Question 23.
Fine the odd one out:
(a) Benzene
(b) Ethane
(c) Ethene
(d) Propyne
Answer:
(a) Benzene
Question 24.
Which among these is not associated with aliphatic compounds?
(a) They contain (4n+2) π electrons
(b) They contain straight chain compounds.
(c) They contain branched-chain compounds.
(d) They have an appropriate number of H-atoms and functional groups.
Answer:
(a) They contain (4n+2) π electron.
![]()
Question 25.
Which of the following compounds will exhibit cis-trans isomerism
(a) 2 – Butene
(b) 2 – Butyne
(c) 1 – Butene
(d) 2 – Butanol
Answer:
(a) 2 – Butene
Question 26.
Which conformation of ethane has the lowest potential energy?
(a) Eclipsed
(b) Staggered
(c) Skew
(d) All will have equal potential energy.
Answer:
(b) Staggered
Question 27.
Arrange the following in the decreasing order of their boiling points
i) n – butane
ii) 2 – methylbutane
iii) n – pentane
iv) 2 – methylbutane
a) i > ii > iii > iv
b) ii > iii > iv > i
c) iv > iii > ii > i
d) iii > ii > iv > i
Answer:
d) iii > ii > iv > i
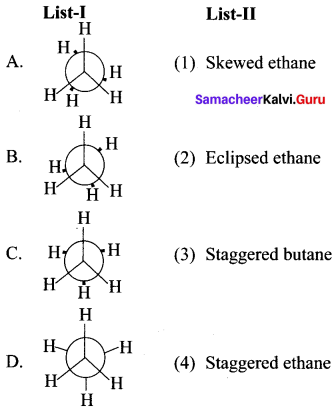
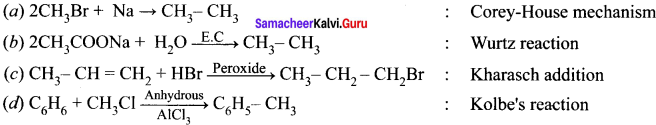
II. Match the following
Question 1.


Answer:
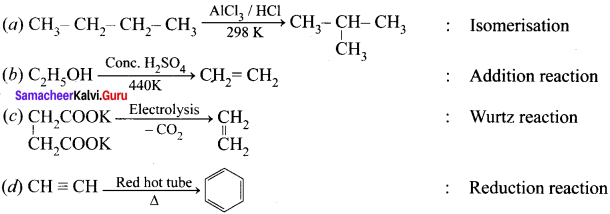
![]()
Question 2.


Answer:
![]()
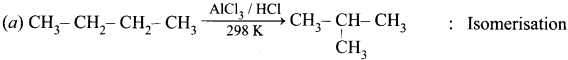
Question 3.

Answer:
![]()
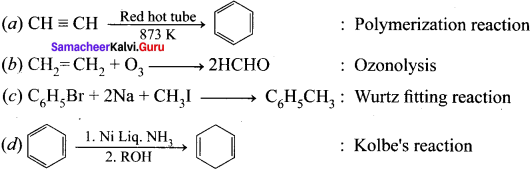
Question 4.


Answer:
![]()
Question 5.


Answer:
![]()
Question 6.

Answer:
![]()
Question 7.
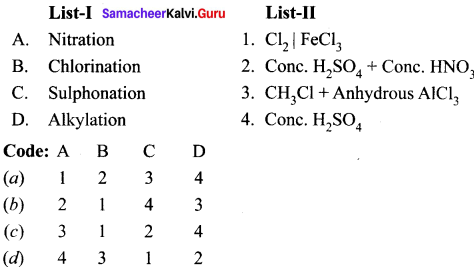
Answer:
![]()
Question 8.
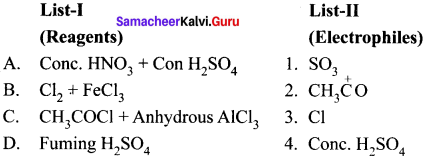

Answer:
![]()
III. Fill in the blanks.
Question 1.
Liquefied petroleum gas consisting of a mixture of ………..
Answer:
Propane + butane
Question 2.
Mangoes contain ………….
Answer:
Cyclo hexane
Question 3.
Methane gas is also called as ………….
Answer:
Marsh gas
![]()
Question 4.
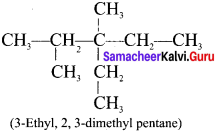
The IUPAC name of the following compound is:
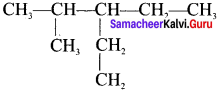
Answer:
3-Ethyl, 2-methylpentane
Question 5.
Sodalime is the mixture of ……………
Answer;
NaOH + CaO
Question 6.
Wurtz reaction used in the preparation of …………
Answer:
higher alkanes
Question 7.
The major reagent present in Corey-House reaction is ………..
Answer:
Lithium dimethyl cuparate
Question 8.
General formula for grignard reagents is ………..
Answer:
R – MgX
Question 9.
The rotation of C – C single bond leads to different isomenc structure called as …………
Answer:
conformers
Question 10.
The least stable conformer of ethane is formed……….
Answer:
eclipsed
Question 11.
The potential energy difference between the staggered and eclipsed conformation of ethane is …………
Answer:
12.5 kJ /mol
![]()
Question 12.
The most stable conformer of butane is ………..
Answer:
Staggered
Question 13.
Paraffin is the older name for the group family of compounds.
Answer:
alkane
Question 14.
Paraffin means ……….
Answer:
Little activity
Question 15.
The preparation of methyl chloride is followed by a mechanism.
Answer:
Free radical
Question 16.
n-hexane passed over chromic oxide supported on alumina at 873 K will give ……….
Answer:
Benzene
Question 17.
The number of possible isomers of C6H12 is …………..
Answer:
5
Question 18.
When ethanol is heated at 440 K with excess of concentrated H2SO4, it will give …………
Answer:
Ethene
Question 19.
Alkynes undergo reduction using Lindlar’s catalyst to give ………….
Answer:
cix – alkenes
Question 20.
Alkynes undergo reduction using sodium in liquid ammonia to give …………
Answer:
trans – alkenes
Question 21.
An aqueous solution of potassium succinate is electrolysed to give ………….
Answer:
Ethane
![]()
Question 22.
The order of reactivity of different hydrogen halides (HCl, HI , HBr) is ………..
Answer:
HI >HBr >HCl
Question 23.
Addition of hydrohalides to alkene is an example for …………
Answer:
Electrophilic additton
Question 24.
Ethane reacts with HBr to form …………
Answer:
Bromo ethane
Question 25.
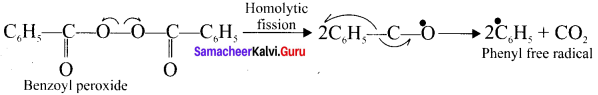
Homolytic fission of benzoyl peroxide will give ………..
Answer:
C6H5
![]()
Question 26.
Propene reacts with HBr in the presence of peroxide to form …………
Answer:
1 – Bromopropane
Question 27.
Baeyer’s reagent is …………
Answer:
alkaline KMNO4
Question 28.
Ozonolysis of ethene produces ………….
Answer:
HCHO
Question 29.
Electrolysis of potassium maleate yields ………
Answer:
Ethyne
Question 30.
Ozonolysis of acetylene gives ………….
Answer:
HCOOH
Question 31.
Three molecules of acetylene undergoes polymerisation to give …………
Answer:
benzene
Question 32.
Benzene reacts with bromine in the presence of AlCl3 to form bromobenzene and it is an example of reaction
Answer:
Electrophilic substitution
Question 33.
The six carbon atoms of benzene are hybridised.
Answer:
sp2
Question 34.
Bond angle in benzene is …………
Answer:
120º
![]()
Question 35.
Benzene contains a bonds and it bonds …………
Answer:
12,3
Question 36.
Wurtz-fittig reaction helps to prepare compounds …………
Answer:
aromatic
Question 37.
When phenol reacts with Zn-dust under dry distillation conditions it gives ………..
Answer:
Benzene
Question 38.
Benzene is insoluble in …………
Answer:
water
Question 39.
Benzene reacts with hydrogen in the presence of Pt to yield ……….
Answer:
Cyclohexane
Question 40.
Benzene reacts with Cl2 in the presence of sunlight to give ………..
Answer:
BHC (Ben.zene hexachioride)
Question 41.
The step in which Cl-Cl bond homolysis occurs is called ……….
Answer:
Initiation step
Question 42.
Dienes are the name given to compounds with ……….
Answer:
Exactly two double bonds
![]()
Question 43.
The hybridisation state of a carbocation is …………
Answer:
sp2
Question 44.
The peroxide effect in anti-markovnikoff addition involves a ………. mechanism.
Answer:
free radical.
IV. Choose the odd one out.
Question 1.
(a) Ethane
(b) Benzene
(c) Ethene
(d) Ethyne
Answer:
(b) Benzene. it is an aromatic hydrocarbon whereas others are aliphatic hydrocarbons.
Question 2.
(a) Zn + HCl
(b) Zn + CH3COOH
(c) LiAlH4
(d) Acidified K2Cr2O7
Answer:
Acidified K2Cr2O7. It is an oxidising agent whereas the other three reagents are reducing
Question 3.
(a) Soft drink bottle
(b) Jars
(c) Vegetable oil bottle
(d) Straws
Answer:
(d) Straws. It is made up of polypropylene whereas others are made of PET (Polyethylene terephthalate).
Question 4.
(a) Straws
(b) Foam cups
(c) Diapers
(d) Toys
Answer:
(b) Foam cups. It is made up of polystyrene whereas others are made up of polypropylene.
Question 5.
(a) Orlon
(b) Neoprene rubber
(c) PVC
(d) PET
Answer:
(d) PET. It is prepared by the polymerization of glycol and terephthalic acid, whereas others are prepared from acetylene.
![]()
Choose the correct pair.
Question 1.
(a) Propene + O3 : HCHO + CH3 CH0
(b) Ethene + O3 : CH3 CHO + H2O2
(c) But-2-ene + O3 : HCHO + H2 O2
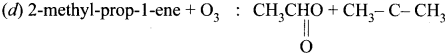
Answer:
(a) Propene + O3 : HCHO + CH3CHO
Question 2.
(a) PET : shampoo bottles
(b) PS : disposable utensils
(c) PP : grocery bags
(d) HDPE : plastic pipes
Answer:
(b) PS : disposable utensils
Question 3.
Answer:
![]()
Question 4.
Answer:
VI. Choose the incorrect pair.
Question 1.
Answer:
![]()
Question 2.
(a) CH2 = CH2 + O3 : 2HCOH
(b) CH3 – CH = CH2 + O3 : CH3CHO + HCHO
(c) CH3 – CH = CH – CH3 + O3 : 2CH3COCH3
(d) CH3 – CH = CH – CH3 + O3 : 2CH3CHO
Answer:
(c) CH3 – CH = CH – CH3 + O3 : 2CH3COCH3
Question 3.
(a) PET: Jars
(b) HDPE: Juice containers
(c) PS: Disposable utensils
(d) PVC: Grocery bags
Answer:
(d) PVC: Grocery bags
![]()
VII. Assertion & Reason.
Question 1.
Assertion (A): Methane is called marsh gas.
Reason (R): Decomposition of plant and animal matter in an oxygen-deficient environment like swamps, marshes and bogs produce methane gas.
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
(b) Both (A) and (R) are correct but (R) is not the correct explanation of (A).
(c) (A) is correct but (R) is wrong.
(d) (A) is wrong but (R) is correct.
Answer:
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
Question 2.
Assertion (A) : Water destroys grignard reagent and so it is not used as solvent for RMgX.
Reason (R) : Water decomposes grignard reagent (RMgX) to give alkane.
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
(b) Both (A) and (R) are correct but (R) is not the correct explanation of (A).
(c) (A) is correct but (R) is wrong.
(d) (A) is wrong but (R) is correct.
Answer:
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
Question 3.
Assertion (A): The boiling point of straight-chain isomers have higher boiling point as compared to branched-chain isomers.
Reason (R): The boiling point decreases with increase in branching as the molecule becomes compact and the area of contact decreases.
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
(b) Both (A) and (R) are correct but (R) is not the correct explanation of (A).
(c) (A)is correct but (R) is wrong.
(d) (A) is wrong but (R) is correct.
Answer:
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
![]()
Question 4.
Assertion (A) : The eclipsed conformation of ethane is less stable than staggered conformation of ethane.
Reason (R) : In eclipsed conformation, the distance between the two methyl group is minimum and so there is maximum repulsion between them and it is the least stable conformer.
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
(b) Both (A)and (R) are correct but (R) is not the correct explanation of (A).
(c) (A) is correct but (R) is wrong.
(d) (A) is wrong but (R) is correct.
Answer:
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
Samacheer Kalvi 11th Chemistry Hydrocarbons 2 Mark Questions and Answers
Question 1.
What are unsaturated hydrocarbons?
Answer:
hydrocarbons having localised carbon-carbon multiple bonds are called unsaturated hydrocarbons.
Example :
alkenes and alkynes.
Question 2.
What is marsh gas?
Answer:
Answer:
- Methane is the major component of the atmosphere of jupiter, Saturn, Uranus and Neptune but only minor component of earth atmosphere.
- Decomposition of plant and animal matter in an oxygen deficient environment like swamps, marshes bogs and the sediments of lakes produces methane gas. It is otherwise known as marsh gas.
Question 3.
Write a note on methane clathrates.
Answer:
- A frozen mixture of water and methane gas is chemically known as methane clathrate.
- The methane molecule which is produced by biological process under the deep -ocean (at 4°C and 50 atm) does not simply reach the surface instead each molecule is trapped inside the clusters of 6 to 18 water molecules forming methane clathrates.
Question 4.
What are alkenes? Give example.
Answer:
Alkenes are unsaturated hydrocarbons that contain carbon-carbon double bond. They are represented by the general formulae CnH2n where ‘n’ stands for a number of carbon atoms in the molecule. Alkenes are also known as olefins (in Latin – oil maker) because the first member ethene combines with chlorine gas to form an oily liquid as a product.
Example:
Ethylene (C2H4)
Question 5.
Draw and name the possible structural formula for C4H10
Answer:
C4H10 has two possible structural formula, they are,
(i) CH3-CH2-CH2-CH3 n-Butane
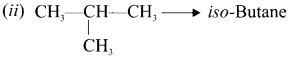
Question 6.
Give the IUPAC name of the following compounds.

Answer:
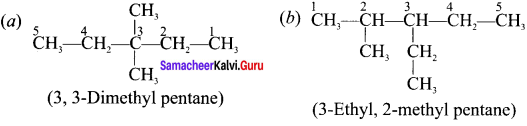
Question 7.
What is Sabatier- Sendersens reaction?
Answer:
The process of addition of H2 to unsaturated compounds (alkenes or alkynes) in known as hydrogenation. The above process can be catalysed by nickel at 298 K. This reaction is known as Sabalier-Sendersens reaction.
For example:
![]()
Question 8.
What are decarboxylation reactions? Given an example.
Answer:
When a mixture of sodium salt of carboxylic acid and sodalime is heated an alkane is formed. During this process CO2 molecule is eliminated process and this process is known as decarboxylation reaction.
![]()
Question 9.
Write a note on Kolbe’s electrolytic method?
Answer:
When potassium or sodium salt of carboxylic acid is electrolysed, a higher alkane is formed.
![]()
Question 10.
How will you prepare propane from Chloropropane?
Answer:
Nascent hydrogen reacts with chloropropane to give propane.
![]()
Question 11.
What is Wurtz reaction?
Answer:
When a solution of haloalkanc in dry ether is treated with sodium metal, higher alkanes are produced. This reaction is known as Wurtz reaction. For example:
![]()
Question 12.
Write shnrt flotes on Corey-House reaction?
Answer:
An alkyl halide and lithium dialkyl cupratc are reacted to give higher alkanes. This reaction is known as Corey-House reaction.
![]()
Question 13.
How will you prepare methane from grignard reagent’?
Answer:
Methyl magnesium chloride reacts with water to give methane.
![]()
Question 14.
What are conformers?
Answer:
The rotation about C-C single bond axis yielding several arrangements of a hydrocarbon called conformers.
![]()
Question 15.
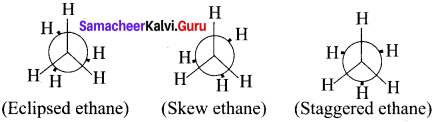
Draw the conformations of ethane using Newman projection formula method?
Answer:
Question 16.
What are combustion reactions?
Answer:
A combustion reaction is a chemical reaction between a substance and oxygen with evolution of heat and light. In the presence of sufficient oxygen alkanes undergoes combustion when ignited and produces carbon dioxide and water.
![]()
Question 17.
What is arornatisation?
Answer:
Alkanes with six to ten carbon atoms are converted into homologous of benzene at higher temperature and in the presence of a catalyst. This process is known as aromatisation. For example, n-Hexane passed over Cr2O3 supported on alumina at 873 K gives benzene.
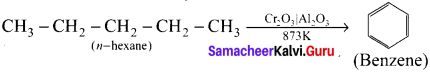
Question 18.
Write notes on isomerisation.
Answer:
1. Isomerisation is a chemical process by which a compound is transformed into any of its isomeric form.
2. Normal alkanes can be converted into branched alkanes in the presence ofAlCl3 and HCl at 298 K.
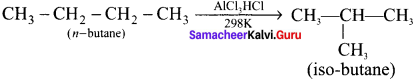
3. This process is of great industrial importance, the quantity of gasoline is improved by isomerising its components.
![]()
Question 19.
Mention the uses of alkanes.
Answer:
- Alkanes are extensively used as fuels.
- Methane present in natural gas is used in home heating.
- A mixture of propane and butane is known as LPG gas which is used for domestic cooking purpose.
- Gasoline is a complex mixture of many hydrocarbons used as a fuel for internal combustion engines.
Question 20.
Draw and name the structural formula for C4H8’?
Answer:
(i) CH3 – CH = CH – CH3 -w 1 – Butene
(ii) CH2 = CH – CH2 – CH2 – 2 Butene
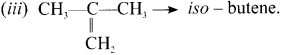
Question 21.
cis-isomers are less stable than rans-isomers?
Answer:
Consider:

Among cis and trans isomers, cis isomer is less stable than trans isomer. In the cis isomer, similar groups arc very near to each other. vander Waal’s repulsion and steric hindrance make the molecule much more unstable. But in trans isomer, similar groups are diagonally opposite to each other and there is no such steric hindrance. Due to more steric interaction cis isomers is less stable than Irons isomer.
Question 22.
How will you prepare ethene from ethanol?
Answrer:
When ethanol is heated at 430 K – 440 K with excess cone. H2SO4. a molecule of water from alcohol is removed and ethene or ethylene is formed.
![]()
Question 23.
How will you convert 1-brornopropane into popene?
Answer:
![]()
1-bromopropane reacts with alcoholic KOH and eliminate hydrogen bromide resulting in the formation of propene.
Question 24.
How will you prepare ethene by Kolbe’s electrolytic method?
Answer:
When an aqueous solution of potassium succinate is electrolysed between two platinum electrodes, ethene is produced at the anode.
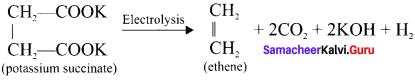
Question 25.
Write any two test for alkenes.
Answer:
- Rapid decolourisation of bromine in CCl4 without evolution of hydrogen bromide.
- Decolourisation of cold dilute aqueous solution of KMnO4
Question 26.
State Markovnikoff’s rule.
Answer:
When an unsymmetrical alkene reacts with hydrogen halide, the hydrogen atom adds to the carbon atom that has more number of hydrogen atoms and halogen add to the carbon atom having fewer hydrogen atoms.
![]()
Question 27.
What is peroxide effect?
Answer:
The addition of HBr to an alkene in the presence of organic peroxide gives the antì-Markovnikofl’s product. This effect is called as peroxide effect.
Question 28.
Identify the products A and B from the following reaction.
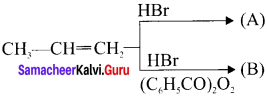
Answer:
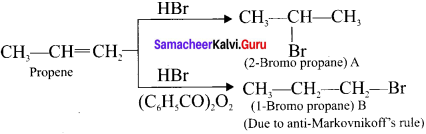
Question 29.
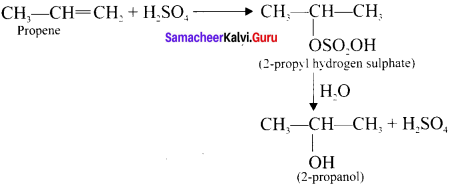
What happens when propcne reacts with concentrated H,S04?
Answer:
Propene reacts rjth cone. H2S04 to form 2 – propyl hydrogen sulphate in accordance with Markovnikoffs rule Further hydrolysis yields 2-propanol.
Question 30.
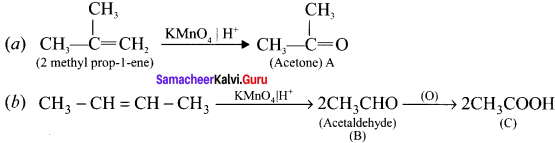
Complete the following reaction and identify A, B and C.
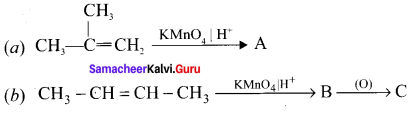
Answer:
Question 31.
Mention the uses of alkenes.
Answer:
- Alkenes are used as starting material in the synthesis of alcohols, plastics, detergents and fuels.
- Ethenc is the most important organic fcedstock in the polymer industry. Examples are, PVC, Sarans and Polythene. These polymers are used in the manufacture of floor tiles, shoe-soles, synthetic fibres, raincoats, pipes etc.
Question 32.
What are gem dihalides? How will you prepare propyne from gem dihalides?
Answer:
Compounds containing two halogen atoms on the same carbon atom are called gem dihalides. On heating 1,1 -dichloropropane with alcoholic KOH, it will give propyne.
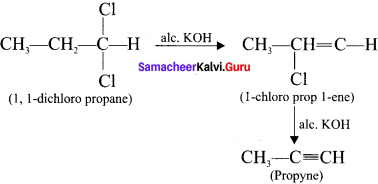
Question 33.
How will you prepare acetylene from potassium maleate?
Answer:
Electrolysis of potassium maleate yields acetylene. This is one of Kolbe’s electrolytic reaction.
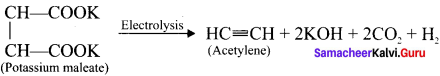
Question 34.
How will you prepare acetylene from calcium carbide?
Answer:
Acetylene can be manufactured in large scale by action of calcium carbide with water.
![]()
Question 35.
How will you convert ethyne into ethanol?
Answer:
Ethyne undergo hydration on warming with mercuric sulphate and dil H2SO4 at 333 K to form ethanol (Acetaldehyde).
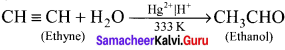
Question 36.
Mention the uses of Acetylene.
Answer:
- Acetylene is used in oxy acetylene torch used for welding and cutting metals.
- It is used for manufacture of PVC, polyvinyl acetate, Polyvinyl ether, orlon and neoprene rubbers.
Question 37.
What are all the conditions for aromaticity?
Answer:
Huckel proposed that aromaticity is a function of electronic structure of an organic compound.
A compound may be aromatic, if it obey the following rules:
- The molecule must have a cyclic structure.
- The molecule must be co-planar.
- Complete delocalisation of it electrons in the ring.
- Presence of (4n + 2) it electrons in the ring where n is an integer (n = 0,1,2….), this is known as Huckel’s rule.
Question 38.
Classify the following compounds by using aromaticity concepts:
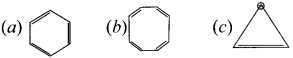
Answer:

- It is co-planar molecule.
- It has six delocalised ir electrons.
- 4n + 26
4n = 6 – 2
4,n = 4
n = 1, obeys Huckel’s rule with n = 1
(b) ![]() Cyclo-octatetraene.
Cyclo-octatetraene.
1. Molecule is non-planar.
Hence it is a non-aromatic compound.
(c)  Cyclopropyl cation.
Cyclopropyl cation.
- It has a co-planar structure.
- It has two delocalised it electrons.
4n + 2 = 2
4n = 0
n = 0
Hence is an aromatic compound.
Question 39.
Write notes on Resonance of benzene?
Answer:
1. The phenomenon in which two or more structures can be written for a substance which has identical position of atoms is called resonance.
2. The actual structure of the molecule is said to be a resonance hybrid of the various possible alternative structures.
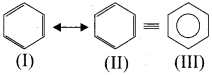
3. In benzene, Kekule’s structures I and II represented the resonance structures, and structure III is the resonance hybrid of structures I and II.
4. The structures I and II exist only in theory. The actual structure of benzene is the hybrid of two hypothetical resonance structures.
![]()
Question 40.
How will you convert phenol into benzene?
Answer:
When phenol vapours are passed over zinc dust then it is reduced to benzene:
![]()
Question 41.
What is Wurtz-fitting reaction?
Answer:
When a solution of bromobenzene and iodomethane in dry ether is treated with metallic sodium, toluene is formed.
![]()
Question 42.
What are activating and deactivating groups?
Answer:
- When mono substituted benzene undergoes an electrophilic substitution reaction, the rate of the reaction and the site of attack of the incoming electrophile depends on the functional group already attached to it.
- Some groups increases the reactivity of benzene ring and are known as activating groups.
- Some groups decreases the reactivity of benzene ring and are known as de-activating groups.
- Example:
Activating groups: – NH2 – OH. – CH3 etc.
Deactivating groups: – NO3. CN, – CHO etc,
Question 43.
Why does benzcnc undergo clectrophilic substitution reactions easily and nucleophilic substitution with difficulty?
Answer:
Due to the presence of an electron cloud containing 6π – electrons above and below the plane of the ring, benzene is a rich source of electrons, Consequently, it attracts the electrophilic reagents towards it and repels the nucleophilic reagents. As a result, benzene undergoes electrophilic substitution reactions easily and nucleophilic substitution with difficulty.
Question 44.
Out of benzene, m – dinitrobenzene and toluene which will undergo nitration most easily and why?
Answer:
CH3 group is an electron-donating group, while -NO2 group is electron withdrawing group. Therefore, maximum electron density will be there in toluene, followed by benzene and it is least in pn-dinitrobenzene. Therefore, the ease of nitration decreases in the order:
Toluene > benzene > m – dinitrobenzene.
Question 45.
Why are alkanes called paraffins?
Answer:
Paraffins means little affinity. Alkanes due to strong C-C and C-H bonds are relatively chemically inert. They are thus called as paraflins.
Samacheer Kalvi 11th Chemistry Hydrocarbons 3 Mark Questions and Answers
Question 1.
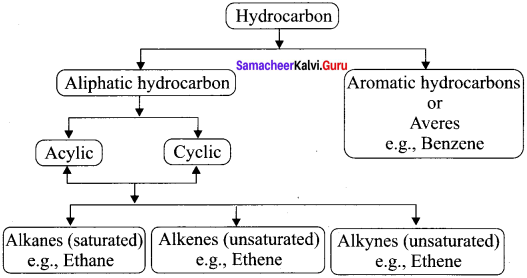
Explain the classification of hydrocarbons.
Answer:
Question 2.
How to write the possible isomers of C5H12?
Answer:
1. To begin draw the carbon backbone of the straight chain isomer.
C-C-C-C-C
2. To determine the carbon backbone structure of the other isomers, arrange the carbon atoms in the other way:
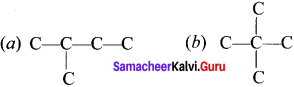
3. Fill in ail the hydrogen atoms so that each carbon forms four bonds,
Question 3.
Explain how to draw the structural formula for 3-ethyl, 2, 3-dimethylpentane.
Answer:
3-ethyl, 2, 3-dimethylpentane.
Step 1:
The parent hydrocarbon is pentane. Draw the chain of five carbon atoms and number
![]()
Step 2:
Complete the carbon skeleton by attaching the alkyl group as they are specified in the name. An ethyl group is attached to carbon atom 3 and two methyl groups are attached to carbon atoms 2 and 3.
Step 3:
Add hydrogen atoms to the carbon skeleton so that each carbon atoms have four bonds.
Question 4.
In alkane compounds with same number of carbon atoms, straight chain isomers have higher boiling point as compared to branched chain isomers-justify statement.
Answer:
- The boiling point of continuous chain alkanes increases with increase in the length of carbon chain roughly about 30°C for every added carbon atom to the chain.
- Alkanes are non-polar compounds and having weak Vander Wal’s force which depends upon molecular surface area and hence increases with increase in molecular size.
- The boiling point decreases with increase in branching on the molecule i.e. as it becomes compact and the area of the contract decreases. Hence in alkanes with same number of carbon atoms, straight chain isomers have higher boiling point as compared to branched chain isomers.
Question 5.
Why oil spills in aqueous environment spread so quickly?
Answer:
- Water molecules are polar and akanes are non-polar. The insolubility of alkanes in water makes them a good water repellent for metals which protects the metal surface from corrosion.
- Because of their lower density than water they tòrm two layers and occupies the top layer. The density difference between alkanes and water explains why oil spills in aqueous environment spread so quickly.
Question 6.
Explain pyrolysis method.
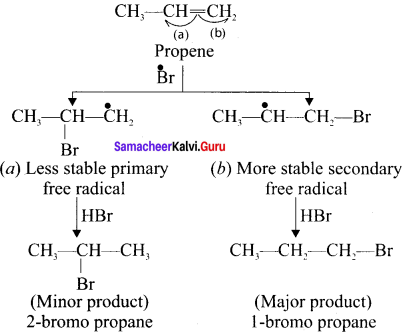
Answer:
1. Pyrolysis is defined as the thermal decomposition of an organic compound into smaller fragments in the absence of air through the application of heat. Pyrolysis of alkanes is also named as cracking.
2. In the absence of air, whai alkane are vapours passed through red-hot metal it breaks into simpler hydrocarbons.
3. The product depends upon the nature of alkane, temperature, pressure and presence or absence of the catalyst.
Question 7.
Write notes on Geometrical isomerism or cis-trans isomerism.
Answer:
1. It is a type of stereoisomerism and it is also called cis-trans isomerism. Such type of isomerism results due to the restricted rotation of doubly bounded carbon atoms.
2. If the similar groups lie on the same side then the geometrical isomers are called as cis-is omers.
3. If the similar groups lie on the opposite side then the geometrical isomers are called as trans-isomers.
Question 8.
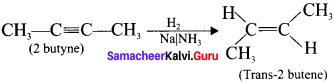
Explain how 2-butyne reacts with (a) Lindlar’s catalyst and (b) Sodium in liquid ammonia?
Answer:
(a) 2-butyne reacts with Lindlar’s catalyst:
2-butyne can be reduced to cis-2 butene using CaCO3 supported in Pd -metal partially deactivated with sulphur.
This reaction is stereo specific giving only the cis-2-butene.

(b) 2-butyne reacts with sodium in liquid ammonia:
2-butyne can also be reduced to trans-2-butene using sodium in liquid ammonia. This reaction is stereospecific giving only the trans-2-butene.
Question 9.
What are vicinal dihalides? How will you prepane alkene from vicinal dihaldes?
Answer:
1. The compounds in which two halogen atoms are attached to adjacent carbon-atoms are called as vicinal dihalides.
2. When vicinal dihalides are warmed with granulated zinc in methanol they lose a molecule
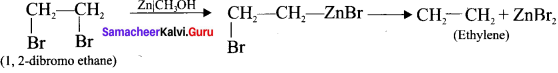
Question 10.
Why alkenes are more reactive than alkanes?
Answer:
1. Alkenes are more reactive than alkanes due to the presence of a double bond.
2. The a-bond is strong but the it-bond is weak. The typical reactions of alkenes involve the addition of an electrophile across the double bond proceeding through ionic mechanism. However addition reactions proceed through free-radical mechanism also. Ozonolysis and nolvmerisation are some of the characteristic reaction of alkenes.
![]()
Question 11.
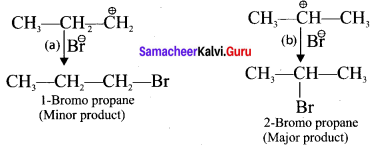
Explain the mechanism of addition of HBr to propenc.
Answer:
Step 1:
Formation of electrophile:
In HBr, br is more electronegative than H. When bonded electron move towards Br, polarity is deve[oped and it creates electrophile H+ which attacks to the double bond to form a carbocation.
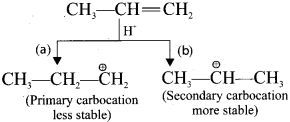
Step 2 :
Secondary carbocation is more stable than primary carhocation and it predominates over the primary carbocation.
Step 3 :
The Br-1 ion attack the 2°- carbocation to form 2-Bromo propane as the major product.
Question 12.
Explain the mechanism of addition of FlBr to 3-methyl-1-butene.
Answer:
Consider the addition of HBr to 3-methyl-1-butene. Here the expected product according to Markovnikoff’s rule is 2-bromo-3-methylbutane but the actual major product is 2-bromo- 2-methylbutane. This is because, the secondary carbocation formed during the reaction is rearranged to give the more stable tertiary carbocation. Attack of Br-1 on this tertiary carbocation gives the major product 2-bromo-2-methylbutane
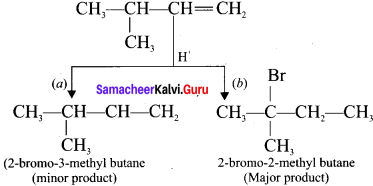
Question 13.
Why peroxide effect is not observed in HCl and HI?
Answer:
The H-Cl bond is stronger (430.5 k.J mol-1) than H-Hr bond (363.7 kJ mol-1), thus H-Cl is not cleaved by the free radical. The H-I bond is further weaker (296.8 kJ mol-3) than H-Cl bond. Thus H-I bond breaks easily hut iodine free radicals combine to form iodine molecules instead of adding to the double bond and hence peroxide effect is not observed in case of HCl and HI.
![]()
Question 14.
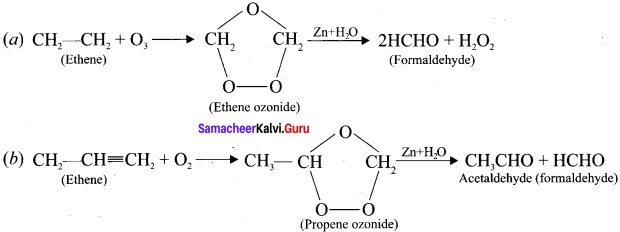
Explain the ozonolysis of (a) Ethene and (b) propene
Answer:
Ozonolysis is a method ofoxidative cleavage of alkenes using ozone and it form two carbonyl compounds. Alkenes react with ozone to form ozonide and it is cleaved by Zn/H2O to form smaller molecules.
This reaction is often used to identify the structure of unknown alkene by detecting the position of double or triple bond.
Question 15.
What is polymerisation? Explain with suitable example.
Answer:
A polymer is a large molecule formed by the combination of large number of small molecules (monomers). This process is known as polymerisation. A few examples are:
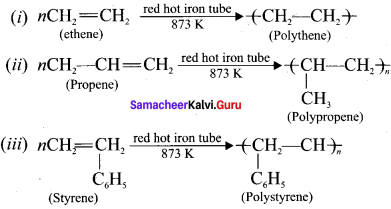
Question 16.
Complete the following reactions:
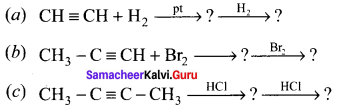
Answer:
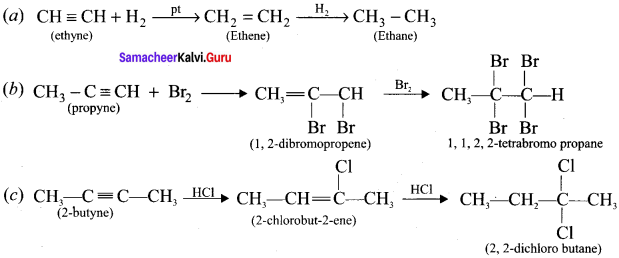
Question 17.
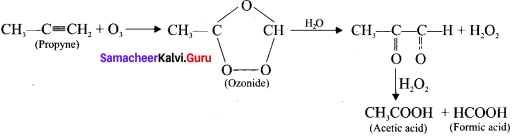
Explain the ozonolysis of (a) Acetylene (b) Propyne
Answer:
Ozone adds to carbon-carbon triple bond of alkynes to form ozonides. The ozonides are hydrolysed by water to form carbonyl compounds. The hydrogen peroxide formed in the reaction may oxidise the carbonyl compound to carboxylic acid.
(a) Acetylene:
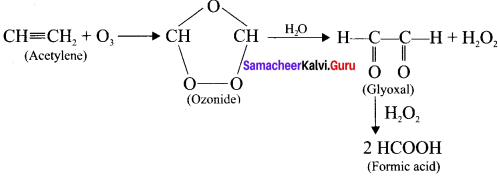
(b) propyne:
Question 18.
Explain the polyrnerisation of acetylene molecules.
Answer:
Acetylene undergoes two types of polymerisation reactions, they are:
- Linear polymerisation
- Cyclic polymerisation
1. Linear polymerisation:
Acetylene forms linear polymer, when passed into a solution of cuprous chloride and ammonium chloride.
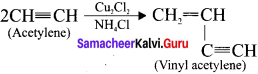
2. Cyclic polymerisation:
Acetylene undergoes cyclic polymerisation on passing through red hot iron tube. Three molecules of acetylene polymerises to form benzene.

Question 19.
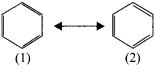
Discuss the Kekule’s structure of benzene.
Answer:
Kekule suggested that benzene consists of a cyclic planar structure of six carbon with alternate single and double bonds.
There were two objections:
1. Bnzene forms only one ortho disubstituted products whereas the Kekule’s structure predicts two ortho disubstituted products as shown:

2. Kekule’s structure failed to explain why benzene with three double bonds did not give addition reaction like alkenes. To overcome this objection, Kekule suggested that henzene was a resonance hybrid of two forms(1 and 2) which are in rapid equilibrium.
Question 20.
Why benzene undergoes substitution reaction rather than addition reactions under normal conditions?
Answer:
1. Each carbon atom in henzene possess an unhybridised p-orbital containing one electron. The lateral overlap of their p-orbitais produces 3π- bond, six electrons of the p-orbitais over all the six carbon atoms and are said lo be delocalised.
2. Due to delocalisation, strong it-bond is formed which makes the molecule stable. Hence unlike alkenes and alkynes, benzene undergoes substitution reactions rather than addition reactions under normal conditions.
![]()
Question 21.
Explain the industrial preparation of benzene from coal tar.
Answer:
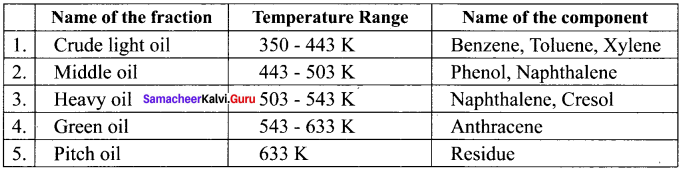
Coal tar is a viscous liquid obtained by the pyrolysis of coal. During fractional distillation, coal tar is heated and distills away its volatile compounds, namely, benzene, toluene, xylene in the temperature range of 350 K to 443 K. These vapours are collected at the upper part of the fractionating column.
Question 22.
Explain the suiphonation of benzene.
Answer:
Benzcne reacts with fuming sulphuric acid and give benzene suiphonic acid. Although SO3 molecule it does not have positive charge, yet it is a strong electrophile. This is because the octet of electrons around the sulphur atom is not reacted. This reached is reversible nd desulphonation occus readily in aqueous medium.
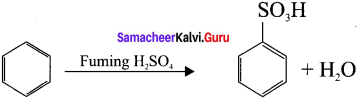
Question 23.
What is BHC? How will you prepare BHC? Mention its uses.
Answer:
1. BHC is I3enzene hexachioride.
2. Benzene reacts with three molecule of Cl2in the presence of sunlight or UV light to yield BHC.
This is also called as gammaxane or Lindane.
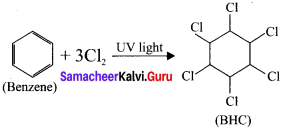
3. BHC is a powerful insecticide.
Question 24.
In aryl halides, halogen group is a oriho -para director and a deactivator towards electrophilic substitution reactions, why?
Answer:
1. In aryl halides, the strong -I effect of the halogens decreases the electron density of benzene ring, thereby deactivating it for electrophilic attack.
2. The presence of lone pair on halogens is involved in resonance with π-electrons of the benzene ring and it increases the electron density at ortho and para position. Hence the halogen group is an ortho-para director and a deactivator.
![]()
Question 25.
Explain the carcinogenity and toxicity of aromatic hydrocarbons.
Answer:
Benzene and polycyclic aromatic hydrocarbons (PAH) are ubiquitous environmental pollutants generated during incomplete combustion of coal, oil, petrol and wood. Some (PAH) originate from open burning, natural seepage of petroleum and coal deposits and volcanic activities. They are toxic, mutagenic and carcinogenic. It has hematological, immunological and neutrological effect on humans They are radioactive and prolonged exposure leads to genetic damage. Some of the examples of PAH arc “L” shaped polynuclear hydrocarbons, which are much more toxic and carcinogenic.
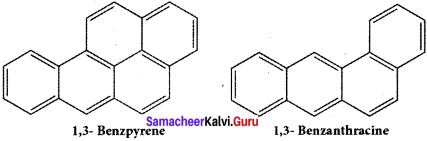
It is found in cigarette smoke, in tobacco and and charcoal boiled food.
It is found in gasoline exhaust and barbecued food.
Question 26.
Arrange benzene, n-hexane and ethyne in decreasing order of acidic behavior. Also give reason for this behavior?
Answer:
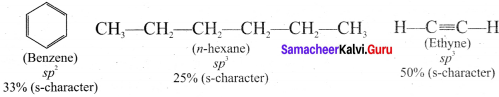
Since s-electrons arc closer to the nucleus, therefore as the s-character of the orbital making the C-H bond increases the electrons of C-H bond lies closer and closer to the carbon atom. In other words, the partial +ve charge on the H-atom and hence the acidic character increases as the s-character of the orbital increases. Thus, the acidic character decreases in the order
Ethyne > Benzene > Hexane
Samacheer Kalvi 11th Chemistry Hydrocarbons 5 Mark Questions
Question 1.
Explain the conformational analysis of ethane.
Answer:
The two tetrahedral methyl groups can rotate about the carbon-carbon bond axis yielding several arrangements called conformers. The extreme conformations are staggered and eclipsed conformations. There can be number of other arrangements between staggered and eclipsed forms and their arrangements are known as skew forms.
Eclipsed conformation:
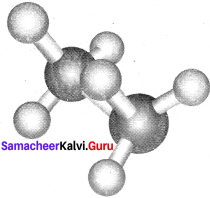
In this conformation, the hydrogen of one carbon atom is directly behind those of the other. The repulsion between the atoms is maximum and it is the least stable conformer.
Staggered conformation:
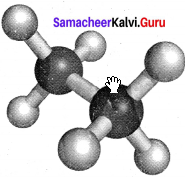
In this conformation, the hydrogen’s of both the carbon atoms are tir apart from each other. The repulsion between the atoms is minimum and it is the most stable conformer.
Skew formation:
The infinite numbers of possible intermediate conformations between the two extreme conformations are referred as skew conformations. The stabilities of various conformations of ethane are
Staggered > Skew> Eclipsed
The potential energy difference between the staggered and eclipsed conformation of ethanc is around 12.5 kJ mol-1HBr The various conformations can be represented by Newman projection formula.
Newman Projection formula for Ethane:
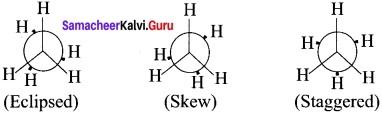
Question 2.
Explain the structure of benzene.
Answer:
1. Molecular formula:
Elemental analysis and molecular weight determination have proved that the molecular formula of benzene is C6H6. This indicates that benzene is a highly unsaturated compound.
2. Straight chain structure is not possible:
Benzene could be constructed as a straight chain but it not feasible since it does not show the properties of alkenes or alkynes. For example, it does not decolorise the bromine water in CCl4.
3. Evidence of cyclic structure:
(1) In the presence of Nickel, benzene reacts with hydrogen to give cyclohexane, a six membered ring. This proves that benzene is a hexagonal
molecule with three double bonds.
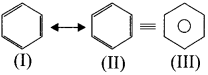
(2) Benzene reacts with bromine in the presence of iron to give substituted C6H5Br. No isomers of C6H5Br was identified. On further reaction with bromine three isomeric disubstituted products C6H4Br2 are formed. On this basis Kekule proposed that benzene consists of ring of carbon atoms with alternate single and double bonds.
4. Resonance description of benzene:
The phenomenon in which two or more structures can be written for a substance which has identical position ofatomsis called resonance. The actual structure of the molecule is said to be a resonance hybrid of various possible alternative structures. In benzene. Kekule’s structure (I) and (II) represented the resonance structures and structure (III) is the resonance hybrid of structure I and II.
5. Spectroscopic measurements:
X-ray and electron dîflìaction studies indicated that all carbon-carbon bonds are of equal length which is in between that of a single bond (1 .45Å ) and that of a double bond (1.34Å ).
6. Molecular orbital structure :
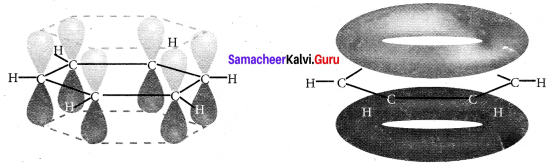
(1). Benzene is a flat hexagonal molecule with all carbons and hydrogen lying in the same plane with a bond angle of 120º. Each carbon atom has sp2 hybrid orbitais of carbon, overlap with each other and with s-orbitals of six hydrogen atoms forming six sigma (σ) C-H bonds and six sigma (σ) C – C bonds.
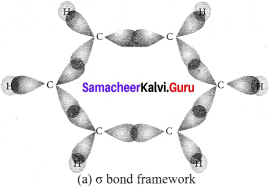
(2). All the σ-bonds in benzene lies in one plane with bond angle of 120º. Each C-atom in benzene possess an unhybridised p-orbital containing one electron. The lateral overlap of their p-orbitais produces 3π-bond, the six electrons of the p-orbitais cover all the six C-atoms and are said to be delocalised. Due to this delocalisation, strong π-bond is formed which makes the molecule stable.
7. Representation of benzene:
Hence, there are three ways is which benzene can be represented.
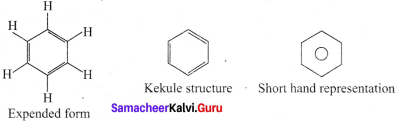
Question 3.
Explain the mechanism of the reaction between methane and chlorine.
Answer:
Methane reacts with chlorine in the presence of light which proceeds through the free radical chain mechanism. This mechanism is characterised by three steps: initiation, propagation and termination.
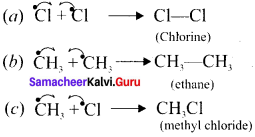
1. Chain initiation:
The chain is initiated by UV light, leading to homolytic fission of chlorine molecules into free radicals (chlorine atoms).
![]()
Here we choose Cl-Cl bond for fission because C- C and C-H bonds are stronger than Cl-Cl bond.
2. Chain propagation:
It proceeds as follows –
(a) Chlorine free radiais attack the methane molecule and breaks the C-H bond resulting in the generation of methyl free radicals.
![]()
(b) The methyl free radicals thus obtained attacks the second molecule of chlorine to give chloromethane (CH3Cl) and a chlorine free radical as follows –
![]()
(c) This chlorine free radical then cycles back to step (a) and both steps (a) and (b) are repeated many times and thus a chain of reaction is set up.
3. Chain termination:
Aller sometime, the reaction stops due to the consumption of reactant and the chain is terminated by the combination of free radicals.
Question 4.
Write the mechanism for following reaction.
![]()
Answer:
![]()
The reaction proceeds through freee radical mechanism:
Step 1.
The weak O-O single bond linkage of peroxide undergoes homolytic cleavage to generate free radical.
Step 2.
The radicals abstracts a hydrogen atom from HBr thus generating bromine free radical.
![]()
Step 3.
The Bromine free radical adds lo the double bond in the way so as to form the more stable alkyl free radical,
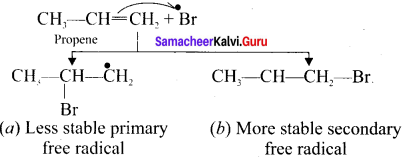
Step 4.
Addition of HBr to secondary free radical.
Question 5.
Explain the acidic nature of alkynes.
Answer:
An alkyiic shows acidic nature only if it contains terminal hydrogen atom. This can be explained by considering the Sp hybrid orbitais of carbon atom in alkynes. The percentage of s-character of sp hybrid orbital (50%) is more than sp2 hybrid orbital ofalkenes (33%) and sp3 hybrid orbital of alkanes (25%). Because of this, carbon atom becomes more electronegative, thus facilitating donation of H ions to bases. So hydrogen attached to triply bonded carbon atoms is acidic in nature.
Question 6.
Write the mechanism of chlorination of benzene.
Answer:
Step 1:
Generation of Cl⊕ electrophile.
AlCl + Cl – Cl⊕ + AlClΘ
Step 2:
Attack of the electrophile on the benzene ring to form arenium ion:
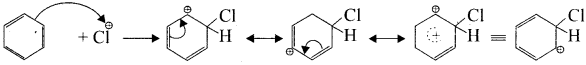
Step 3:
Rearomatisation of arenium ion:
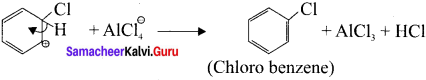
Question 7.
Describe the mechanism of suiphonation of benzene.
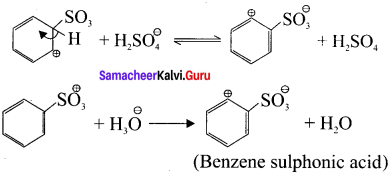
Answer:
Step 1:
Generation of SO3 electrophile:
2H2SO4 → H3O⊕ + SO3 + HSO4Θ
Step 2:
Attack of the electrophile on benzene ring to form arenium ion:
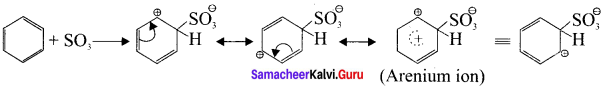
Step 3:
Rearomatisation of Areniurn ion:
Question 8.
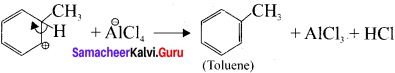
Describe the mechanism of Freidel craft’s alkylation.
Answer:
Step 1:
Generation of ⊕CH3 electrophile:
AlCl3 + CH3Cl → ⊕CH3 + AlClΘ
Step 2 :
Attack of the electrophile on benzene ring to form areniurn ion:
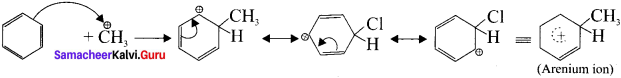
Step 3:
Rearornatisation of arenium ion:
Question 9.
Write the mechanism of Freidel craft’s acylation.
Answer:
Step 1:
Generation of CH3CO electrophile:
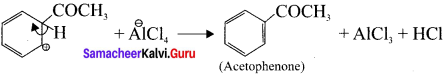
![]()
Step 2 :
Attack of the electrophile on benzene ring to form arenium ion.
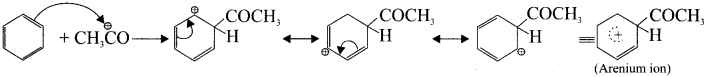
Step 3 :
Rearomatisation of arenium ion:
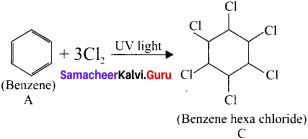
Question 10.
An organic compound (A) of a molecular formula C6H6 which is a simple aromatic hydrocarbon. A undergoes hydrogenation to give a cyclic compound (B). A reacts with chlorine in the presence of UV-light to give C which is used as insecticide. Identify A, B and C. Explain the reactions with equation.
Answer:
1. Simple aromatic hydrocarbon, C2H6 is benzene.
2. Benzene (A) reacts with H2 in the presence of Pt to give cyclohexane (B).
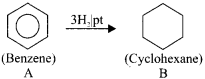
3. Benzene (A) reacts with Cl2 in presence of UV-light to give benzene hexachioride (C).
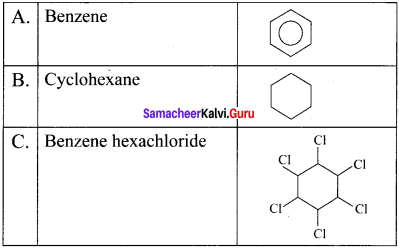
Question 11.
What are ortho-para directors? Explain why -OH group is an ortho-para director and activator.
Answer;
The group which increases the electron density at ortho and para positions are called as ortho-para directors,
Example:
-OH. -NH2 -NHR. -OCH3, -CH3, -C2H5 etc.
Let us consider the directive influence of phenolic group. Phenol is the resonance hybrid of the following structures:
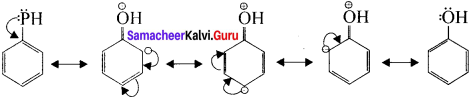
In these resonance structures, the negative charge residue in the present at orilio and para positions of the ring structure. h is quite evident that the lone pair of electrons on the atom which is attached lo the ring is involved in resonance and it makes the ring more electron rich than benzene.
The electron density at oriho and para position increases as compared to the meta position. Therefore phenolic group activates the benzene ring for electrophilic attack at oriho and para positions. Hence 01-1 group is an orilio para director and activator.
Question 12.
An organic compound (A) of a molecular formula C6H6 is a simple aromatic hydrocarbon. A reacts with O2 in the presence of VO5 at 773 K to give B. A is further treated with sodium and liquid ammonia to give C which is a dienc compound. Identify A, B, and C and explain the reactions.
Answer:
1. A is benzene (C6H6), a simple aromatic hydrocarbon
2. Benzene (A) reacts with O2 is the presence of V2O5 at 773K to give maleic anhydride (B).
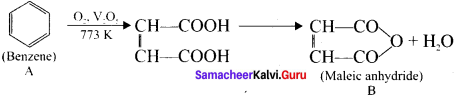
3. Benzene (A) is treated with sodium and liquid ammonia to give 1.4 – cyclohexadiene (C)
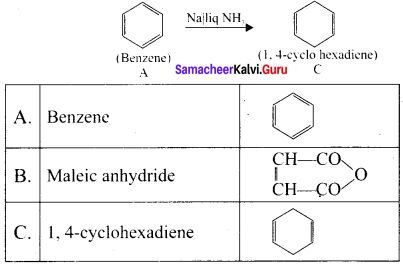
Question 13.
What are inea drectors’? Explain with suitable example.
Answer:
The group which increases the electron density at mcta position are called as rneta directors.
For example:
-NO2. -CN, -CHO, -COOH. -SO3H etc.
Let us consider the directive influence of aldehydic (-CHO) group. Benzaldehyde is a resonence hybrid of the following structures.
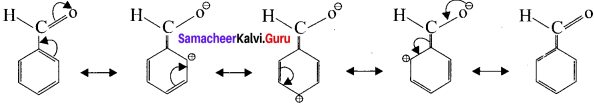
In these resonance structures, the positive charge residue is present on the ring structure. It is quite evident that resonance deLocalises the positive charge on the atoms of the ring, making the ring less electron rich than henzene. Here overall electron density of benzene ring decreases due to -I effect of’ -CHO group, thereby deactivating the henzene for electrophilic atlack. However resonating structures shows that electron density is more at meto position as compared to ortho and para positions. Flence -CHO group is a ineta -director and a deactivator.
![]()
Question 14.
An organic compound (A) of molecular formula C2H4 which is a simple alkene reacts with Baeyer’s reagent to give B of molecular formula C2H6O2 A again reacts with ozone followed by hydrolysis in the presence of zinc to form C of molecular formula CH2O. Identify A, B and C. Explain with reactions.
Answer:
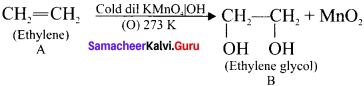
(i). A is CH2 = CH2 (Ethylene)
(ii). A (ethylene) reacts with Baeyer’s reagent (cold alkaline KMnO4) to give ethylene glycol (ethane 1 ,2 diol).
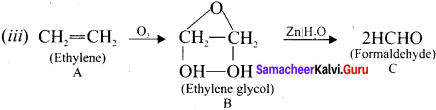
Question 15.
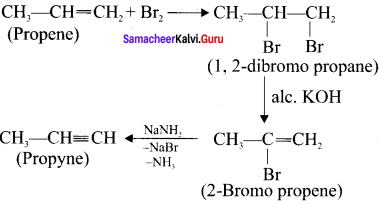
An organic compound 1,1-dichioropropane reacts with aicholic KOH to give A of molecular formula C3H4. A reacts with mercuric sulphate and dil. H2SO4 at 333 K to give B. A on passing through red hot iron tube at 873 K will give C which is a cyclic compound. Identify A, B and C. Explain the reactions.
Answer:
1. 1,1-dichioropropane reacts with aicholic KOI-I to give propyne (A).
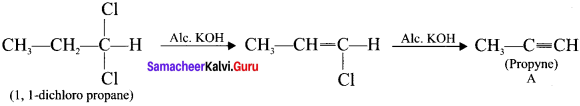
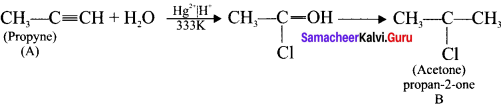
2. Propyne (A) reacts with mercuric sulphate and dil. H2SO4 at 333 K to give acetone (B).
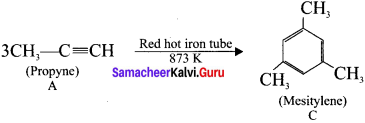
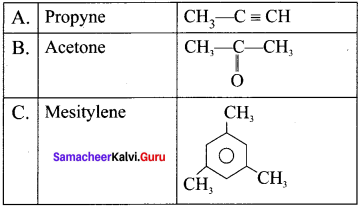
3. Propyne (A) on passing through red hot iron tube at 873 K will give mesitylene (C).