You can Download Samacheer Kalvi 7th Science Book Solutions Guide Pdf, Tamilnadu State Board help you to revise the complete Syllabus and score more marks in your examinations.
Tamilnadu Samacheer Kalvi 7th Science Solutions Term 2 Chapter 3 Changes Around Us
Samacheer Kalvi 7th Science Changes Around Us Textual Evaluation
I. Choose the best answer :
Question 1.
When a woolen yarn is knitted to get a sweater, the change can be classified as _________
(a) physical change
(b) chemical change
(c) endothermic change
(d) exothermic change
Answer:
(a) physical change
Question 2.
_________ of the following are endothermic changes.
(a) Condensation and melting
(b) Condensation and freezing
(c) Evaporation and melting
(d) Evaporation and freezing
Answer:
(a) Condensation and melting
Question 3.
The chemical change is _______
(a) water to clouds
(b) growth of tree
(c) cow dung to bio-gas
(d) ice-cream to molten ice-cream
Answer:
(c) cow dung to bio-gas
Question 4.
_______ is an example of a periodic change
(a) Earthquake
(b) Formation of rainbow in sky
(c) Occurrence of tides in seas
(d) Showering of rain
Answer:
(a) Earthquake
![]()
Question 5.
_______ is not a chemical change.
(a) Dissolution of ammonia in water
(b) Dissolution of carbon-dioxide in water
(c) Dissolution of oxygen in water
(d) Melting of polar ice caps
Answer:
(b) Melting of polar ice-caps
II.Fill in the blanks :
- Filling up a balloon with hot air is a _______ change.
- Stretching gold coin into a ring is a _______ change.
- Opening a gas cylinder knob converts _______ fuel into _______ fuel. This is an example of _______ change.
- Spoiling of food is a _______ change.
- Respiration is a _______ change.
Answer:
- physical
- physical
- liquid, gaseous, chemical
- chemical
- exothermic chemical
III. True or False – If False give the correct answer :
Question 1.
Cutting of cloth is an example of a periodic change.
Answer:
False, Cutting of cloth is an example of a physical change.
Question 2.
Taking a glass of water and freezing it by placing it in the freezer is a chemical change.
Answer:
False, Taking a glass of water and freezing it by placing it in the freezer is a physical change
Question 3.
A bean plant collecting sunlight and turning it into bean seeds is an example of physical and non-periodic change.
Answer:
False, A bean plant collecting sunlight and turning it into bean seeds is an example of chemical and non-periodic change.
Question 4.
If the chemical properties of a substance remain unchanged and the appearance or shape of a substance changes it is called a periodic change.
Answer:
False, if the chemical properties of a substance remain unchanged and the appearance or shape of a substance changes it is called a fossil change.
![]()
Question 5.
Tarnishing of silver is an example of endothermic change.
Answer:
False, Tarnishing of silver is an example of change.
IV. Match the following:
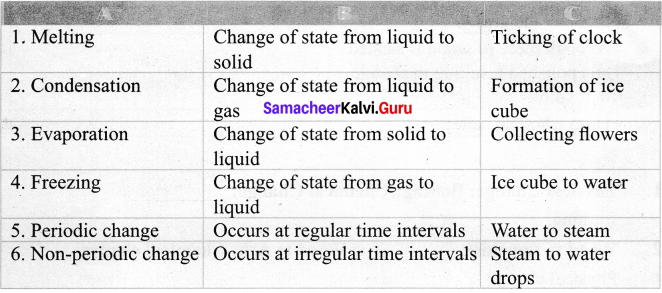
Answer:
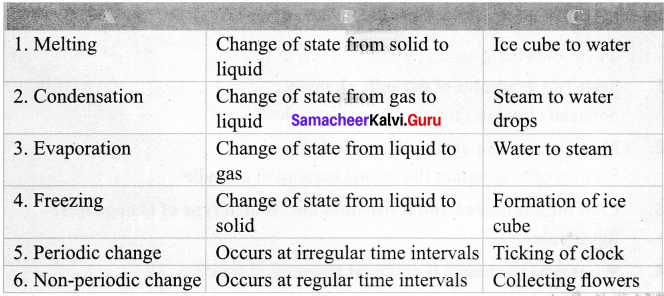
V. Classify the following changes as physical and chemical changes:
Question 1.
A rough piece of wood is sanded and polished resulting in change in texture, Rusting of a iron nail, Painting the grill, Bending a paper clip, Pounding silver into thin plate, Rolling the chapathi dough into thin wire, Occurrence of day and night, eruption of volcano, burning of matchstick, dosa from the batter, blinking of eyelids, occurrence of a thunderstorm, rotation of the earth, formation of eclipses.
Answer:
Physical changes
- Bending a paper clip.
- Pounding silver into thin plate.
- Rolling a chapathi dough into thin wire.
- Occurrence of a day and night.
- Blinking of eyelids.
- Occurrence of a thunderstorm.
- Rotation of the earth.
- Formation of eclipses.
- Painting the grill.
- A rough piece of wood is sanded and polished resulting in change in texture.
- Dosa from the batter.
Chemical changes
- Rusting of a iron
- Eruption of volcano
- Burning of matchstick.
VI Analogy:
Question 1.
Physical Change: Boiling::Chemical Change: ________
Answer:
Burning.
Question 2.
Wood to saw dust: _________ :: Wood to Ash: Chemical change
Answer:
Physical change.
![]()
Question 3.
Forest fire: ________ change::Change in period in a school: periodic change
Answer:
Non-periodic.
VII. Very short answer type question :
Question 1.
State two examples of periodic changes.
Answer:
Seasonal changes, motion of hands of a clock.
Question 2.
Mention any two exothermic reactions.
Answer:
Burning of magnesium ribbon and burning of a candle.
Question 3.
Cold milk is heated and it becomes hot. Which type of change it is?
Answer:
Reversible.
Question 4.
What type of change is artificial ripening of fruit?
Answer:
Irreversible chemical change.
![]()
Question 5.
What type of change is coloring of a paper?
Answer:
Physical change.
Question 6.
Growing of nails is a periodic change. Why?
Answer:
Growing of nail is a periodic change, because it occurs periodically at regular intervals.
Question 7.
What type of energy changes is associated when ice melts?
Answer:
![]()
- Physical change
- Endothermic (heat energy is absorbed)
VII. Short answer type question :
Question 1.
Distinguish physical and chemical changes.
Answer:
Physical changes:
- No new substance is formed
- Reversible
- Change in physical properties like size, shape, state
- Melting of ice, tearing of paper freezing, evaporation vaporization
Chemical changes:
- New substance is formed
- Irreversible
- Change in properties of reactants and products
- Burning of paper, photosynthesis, digestion of food, rusting of iron
Question 2.
How can a change occur in a substance?
Answer:
A change can occurs in a substance by an alteration in the properties such as colour, texture and the state of the substance since there is formation of a new substance.
![]()
Question 3.
Can you suggest a method to collect water from sea water?
Answer:
Evaporation.
Question 4.
Is solar eclipse a periodic change? Give your reason.
Answer:
Yes, solar eclipse is a periodic change as it occurs after a definite interval of time.
Question 5.
What is the difference between dissolution of sugar and burning of sugar ?
Answer:
Dissolution of sugar:
- When sugar is dissolved in water it disappears. It we taste the solution, the sugar is still present in dissolved form.
- If water is evaporated we get back the sugar.
- So it is a physical and reversible change.
Burning of sugar:
- Fire activates a chemical reaction between sugar and oxygen. The oxygen in the air reacts with the sugar as the chemical bonds broke.
- Energy is released in the form of smoke.
- So, burning a sugar is a chemical change.
IX. Long answer type question :
Question 1.
Explain the following statement: Digestion is a chemical change.
Answer:
- When we eat, our mouth physically break down food into small pieces.
- Mechanical digestion occurs in the mouth, stomach and small intestine.
- Food is chemically changed in digestion when new, smaller substances are formed.
- Moreover, we will never be able to get back the raw material in the same form as it was before.
- Digestion of food is a permanent change which is irreversible.
![]()
Question 2.
How the iron blade is fixed into a wooden handle in tools used to dig the soil?
Answer:
- First the ring in the iron blade of spade is heated.
- Heating of the blade leads to its expansion and thus the ring gets bigger.
- This happens because metals always expand on heating.
- The metal blade is then fitted easily into the wooden handle.
- After this, cold water is usually poured on the iron blade which leads to the contraction of the expanded iron blade.
- The spade can also be left to cool down, undisturbed so that it is firmly fixed, (vu) The blade is attached firmly to the wooden handle of a spade in this process.
X.Higher order Thinking questions :
Question 1.
Peeled and unpeeled banana does not look the same. Does that mean peeling banana is a chemical change?
Answer:
No, it is not a chemical change. We just separated the skin of the banana and there is no change is its composition..
Question 2.
A very hot glass on putting in cold water cracks. What does this change indicate?
Answer:
When hot glass is cooled fast, the glass cools down unevenly and therefore cause the glass to crack because inside contracts while the outside remains expanded. Glass expands when hot and contracts when cold. It’s a physical but irreversible change.
Question 3.
Boiling of water is a physical change; but boiling of egg is a chemical change. Why?
Answer:
Boiling of water is a physical change:
- On boiling, water is converted into steam. If we cover the beaker with a lid, steam condenses back to water. So, boiling, of water is a physical change since only the physical state of water changes.
Boiling of egg is chemical change:
- When egg is boiled, chemical nature of the egg changes. The properties of a boiled egg are totally different from the raw egg. Hence, boiling of an egg is a chemical change.
XI. Assertion – Reason type question :
Question 1.
Assertion (A) : The explosion of fire cracker is a physical change.
Reason (R) : A physical change is a reversible change.
Option:
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Answer:
(d) A is false but R is true
![]()
Question 2.
Assertion (A) : e process of conversion of liquid water to its vapours by heat mg the liquid is called boiling.
Reason (R) :The process of conversion of water vapours to liquid by cooling the vapors is called condensation
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are 11 but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Answer:
(b) Both A and R are true but R is not the correct explanation Of A.’
Question 3.
Assertion (A) : Burning of wood log to charcoal is a physical change.
Reason (R) : The products formed of burning a piece of wood can be easily converted back to wood log.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Answer:
The correct answer is A Both A and R are false]
Question 4.
Assertion (A) : The formation of iron oxide from iron is a chemical change.
Reason (R) : For the rust to form from iron, it must be exposed to air and water.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false. .
(d) A is false but R is true.
Answer:
(a) Both A and R are true and R is the correct explanation of A.
XII. Picture based Questions:
Observe the picture and list down the changes that are accompanied in the picture.

Answer:
(a)Physical change
(b)Chemical change
(c) Exothermic
Question 2.
Observe the picture containing a kettle and note that it has salt water in it and answer the following questions.
(a) What is name of the process that is done to the kettle?
(b) What will happen to the content of the kettle?
(c) What kind of change is occurring on the cold surface of the metal plate?
(d) What can you say about the quality of water that is obtained in the beaker?
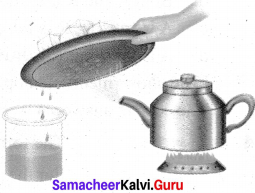
Answer:
(a) Boiling
(b) Water in the kettle gets converted into vapour
(c) Endothermic
(d) Water vapour converted into liquid (condensation)
![]()
Samacheer Kalvi 7th Science Changes Around Us Intext Activity
Activity – 3
Question 1.
Take two pans, one wide and another narrow. Fill hot water in both to the same depth. Keep them in open. Observe after one to two hours. The pan that is wide has cooled more than the narrow one. That is more the surface area; the rate of evaporation is more.
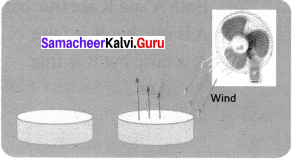
From this, can you guess why we unfurl the clothes while putting them to dry, rather than just drape them over the cloth line?
Answer:
Wet clothes dry faster when we unfurl (Spread) them because the rate of evaporation depends on the surface area. If the surface area will be more, rate of evaporation will be higher or more.
Activity – 7
Question 1.
Take a small piece of magnesium ribbon and clean it by rubbing its surface with a sand paper. Hold the magnesium ribbon at one end with a pair of tongs and bring its other end over the flame of a burner.
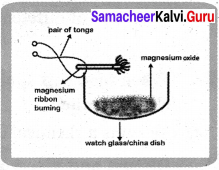
Answer:
Magnesium ribbon must be cleaned before burning. So that the layer of magnesium oxide can be removed in order to get the desired chemical reaction.
Magnesium ribbon burns in air with a dazzling flame and forms a white ash, I magnesium gets oxidised to magnesium oxide.
Activity- 9
Question 11.
Ask a student to stretch both hands, put a pinch of soap powder in one hand and a pinch of glucose in the other hand. Add a few drops of water to soap powder and ask how the student feels upon adding water. Now add a few drops of water to the glucose at the other hand. Now ask the student how he /she feels on adding water, What is the feeling when water is added to glucose?
Answer:
We feel cool.
Question 10.
What is the difference when water is added to soap powder and when water is added to glucose?
Answer:
When water is added to soap powder we feel hot. When water is added to glucose we feel cold.
![]()
Intext Questions.
Question 1.
Look at the following list. Identify the physical and chemical changes and fill in the given table.
(rusting of iron, digestion of food, boiling egg, rotting banana, mixing sand and water, chopping wood, crushing a can, mixtures of different coloured buttons, burning of wood)

Answer:
Physical changes:
- Chopping wood
- Mixing sand and water
- Crushing a can
- Mixtures of different coloured buttons
Chemical changes:
- Rusting of iron
- Digestion of food
- Boiling egg
- Rotting banana
- Burning of wood
Question 2.
When food gets spoiled, it produces a foul smell. Shall we call this change as a chemical change?
Answer:
Yes, it is a chemical change.
Question 3.
Discuss and give your answer. You know that plant produce their food by a process called photosynthesis. Can we call photosynthesis a chemical change?
Answer:
Yes.
- Photosynthesis is the process through which plants convert light energy into chemical energy. Here is the chemical reaction involved.
- As we can see, water and carbon di oxide combine to form glucose and oxygen.
- Since new chemical species are formed, photosynthesis is clearly a chemical change.
Samacheer Kalvi 7th Science Changes Around Us Additional Questions.
I. Choose the correct answer
Question 1.
The change of state of a substance from solid to liquid and liquid to gas is ____________
(a) physical change
(b) a chemical change
(c) combination of a physical and chemical changes
(d) none
Answer:
(a) physical change
Question 2.
Rusting of iron is ____________
(a) an irreversible chemical change
(b) a reversible chemical change
(c) an irreversible chemical change
(d) a reversible physical change
Answer:
(a) an irreversible chemical change
Question 3.
Keeping a stone in sunlight for few hours is ____________
(a) a physical change
(b) a chemical change
(c) neither physical nor a chemical change
(d) combination of physical and chemical changes
Answer:
(c) neither physical nor a chemical change
![]()
Question 4.
Beating an egg to make a cake is a ____________
(a) physical change
(b) reversible change
(c) chemical change
(d) change in state
Answer:
(c) chemical change
Question 5.
Large crystals of pure substances can be obtained from their solutions by the process of ____________
(a) sublimation
(b) evaporation
(c) melting
(d) crystallization
Answer:
(d) crystallization
Question 6.
Which of the following is not a physical change?
(a) crushing of a paper
(b) Burning of a paper
(c) making boat of a paper
(d) melting of butter
Answer:
(b) Burning of a paper
Question 7.
Cut vegetables turn brown when exposed to air, this is due to ____________
(a) evaporation
(b) oxidation
(c) neutralization
(d) displacement
Answer:
(b) oxidation
Question 8.
Which gas is produced when vinegar reacts with baking soda?
(a) Hydrogen
(b) carbondioxide
(c) carbon monoxide
(d) oxygen
Answer:
(b) carbondioxide
Question 9.
Vanaspathi is obtained from vegetable oils by addition of ____________ to the oils.
(a) oxygen
(b) hydrogen
(c) carbon di oxide
(d) nitrogen
Answer:
(b) hydrogen
Question 10.
The simplest method of preventing rusting of iron is to coat it with oil, grease or paint. The reason being ____________
Answer:
(a) this layer does not allow iron to come in contact with air.
(b) this layer does not allow iron to come in contact with water
(c) this layer does not allow iron to come in contact
(d) this layer does not allow iron to come in contact with air and water.
Answer:
(c) this layer does not allow iron to come in contact
II. Fill in the Blanks.
- When water is added to _______ there will be evolution of heat along with the formation of slaked lime.
- A lump of curd is the _______ that is obtained by the chemical reaction between hot milk and lemon juice.
- Heat may evolved or absorbed during a _______ change.
- Salt is obtained from sea water by the process of _______
- When magnesium is burnt in air, a new substance is formed which is _______
- _______ occurs in the absence of air and in the presence of micro-organisms such as yeast.
- _______ are substances that speed up the process of a chemical change and it will not under go any change during the course of the reaction.
- Galvanisation is a process is which _______ is coated as a layer on iron.
- The chemical formula of rust is _______.
- _______ happens when molecules in a gas cool down.
- Solid substances like_______ , _______ heating without becoming liquid.
- Dissolution of glucose in water is an _______ Change
- In an endothermic process, the speed of the molecules is _______ hence they move faster
- _______ is the changing of a liquid into its solid state and it happens by cooling.
- The rate of evaporation _______ with rising temperature.
Answer:
- Quicklime
- precipitate
- chemical
- evaporation
- magnesium oxide
- fermantation
- catalysts
- chromium or zinc
- fe2O3.H2O
- condensation
- camphor,naphthalene
- endothermic
- incresed
- freezing
- increses
III. True or False – if false give the correct statement.
Question 1.
Nitrogen gas turns lime water milky.
Answer:
False.
Carbondioxide gas turns lime water milky.
![]()
Question 2.
Cutting a log of wood into pieces is a chemical change.
Answer:
False.
Cutting a log of wood into pieces is a physical change.
Question 3.
Iron pipes coated with zinc do not get rusted easily.
Answer:
True.
Question 4.
Iron and rust are the same substances.
Answer:
False.
Rust is iron oxide.
Question 5.
The chemical name of baking soda is sodium bicarbonate.
Answer:
True.
Question 6.
Adding sugar to milk is a physical change.
Answer:
True.
Question 7.
Magnesium + oxygen → magnesium oxide.
2Mg + O2 → 2Mg O
Answer:
True
Question 8.
Evaporation is a fast process and occurs only at the surface of the liquid.
Answer:
False. Evaporation is a slow process and occurs only at the surface of the liquid.
Question 9.
The rate of evaporation is more when the surface area is greater.
Answer:
True.
![]()
Question 10.
When lemon juice is mixed with soda water, they produce brisk effervescence
Answer:
true
IV. Match the following :
Question 1.
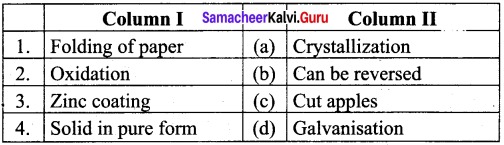
Answer:
- (a)
- (c)
- (d)
- (b)
Question 2.
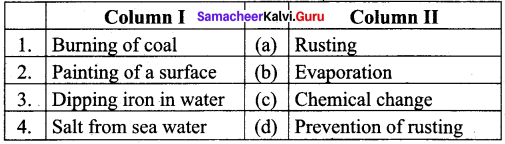
Answer:
- (c)
- (d)
- (a)
- (b)
Question 3.
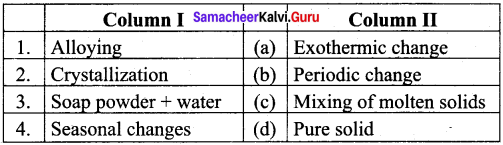
Answer:
- (c)
- (d)
- (a)
- (b)
IV. Very short Answers:
Question 1.
Mention few physical properties of a substance.
Answer:
Lustre, malleability, ductility, density, viscosity, solubility mass and volume.
![]()
Question 2.
What are crystals?
Answer:
Crystals are solids in their purest form that have definite geometrical shapes.
Question 3.
Is conversion of milk into ice-cream a chemical change?
Answer:
Yes, conversion of milk into ice-cream is a chemical change. Since properties of ice-cream are different from the milk
Question 4.
Which is used as a catalyst during the process of hydrogenation of oils?
Answer:
Nickel, platinum or palladium.
Question 5.
Mention some of the different conditions needed for chemical changes to occur.
Answer:
Physical contact of the substances, heat, light, electricity and pressure.
Question 6.
Write the chemical reaction of baking soda with lemon.
Answer:
Sodium hydrogen carbonate + citric acid → sodium citrate + carbon di oxide+ water.
Question 7.
Give examples for endothermic process.
Answer:
Melting, vaporization and sublimation.
Question 8.
Give examples for exothermic process.
Answer:
Freezing and condensation.
Question 9.
Write the equation for the process of formation of rust.
Answer:
Iron + oxygen + water → rust
2Fe + 2O2 + 2H2O →2Fe2O3.H2O
Question 10.
What will happen when we mix baking soda with lemon juice?
Answer:
When we mix baking soda with lemon juice we can hear a hissing sound when bubbles of carbon di oxide coming out and rising is the reaction vessel.
VI. Short Answer.
Question 1.
How would you show that settling of curd is a chemical change?
Answer:
When milk is set to curd, the properties of milk are completely changed and a new product is formed. Curd cannot be converted back to milk, hence it is a chemical change.
Question 2.
Explain how painting of an iron gate prevents it from rusting.
Answer:
When an iron surface is painted, iron does not come in contact with air or moisture. Paint acts as a protective layer and prevents exposure of iron to the atmosphere. Since air and water are necessary for rusting, iron does not get rusted, if painted.
![]()
Question 3.
Give an example where heat, light and sound are produced during a chemical change.
Answer:
Burning of a fire cracker is accompanied by evolution of heat, light and sound.
Question 4.
Give an example of a physical change in which the colour of the substance changes.
Answer:
Heat a piece of iron wire on fire. After some time, it turns red in colour. On cooling it is again converted into its original colour.
Question 5.
Write five changes in the characteristics of a substance which can take place during a chemical change. .
Answer:
- Change in colour
- Change in physical state.
- Evolution of gas
- Absorption or evolution of heat
- Formation of a precipitate
Question 6.
How can we prevent rusting?
Answer:
Iron articles can be prevented from making contact with oxygen, water/water vapour. A simple way is to apply a coat of paint or grease. These coats should be applied regularly to prevent rusting.
Question 7.
Write a note on catalyst.
Answer:
Catalysts are substances that speed up the process of a chemical change and it will not undergo any change during the course of the reaction. For example, yeast acts as the catalyst in the fermentation of sugar.
Question 8.
We are advised to not to play with fireworks. Give reason.
Answer:
Explosion of a firework is a chemical change. This explosion produces heat,Tight, sound andampleasant gases that pollute the atmosphere.
Question 9.
The process of eveporation is not a good technic to seperate salt from a sea water give a reason
Answer:
The process of evaporation is not a good technique because the soluble impurites do not get removed in the process of evaporation.
Question 10.
Chemical changes are important is our day to day life. Give examples
Answer:
- Medicines are prepared by carrying out a chain of chemical changes.
- The materials such as plastics, soaps, detergents, perfumes, acids, bases, salts etc are all made by carrying out various types of chemical changes.
Question 11.
A puddle of water getting pooled around the glass of ice-cream or a glass of ice cubes. When it is kept in room temperature. Give reason.
Answer:
The ice kept in the beaker receives heat from the surrounding air, to melt and form water.
VII. Long Answers
Question 1.
Distinguish between the characteristics of solid, liquid and gas.
Answer:
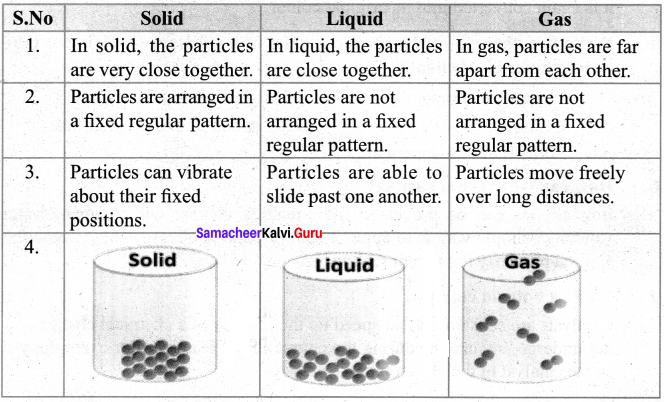
Question 2.
We would have observed that the plate that covers the cooked food items have water droplets inside. Why? Explain.
Answer:
- The water vapour emerges from the hot food and goes up.
- The plate covering the food item is in relative less temperature than the hot food.
- Thus the more energetic molecules loose energy once they touch the cooler plate.
- As the molecules lose heat, they lose energy and slow down.
- They move closer to other gas molecules.
- Finally these molecules collect together to form a liquid.
- Condensation happens when molecules in a gas cool down.
Question 3.
Write note on the following:
- Curdling of milk
- Fermentation
Answer:
(i) Curdling of milk: Curdling is a process in which liquid gradually turns into solid, forming clumps along the way. Take hot milk in a pan and add few drops of curd, in few minutes milk curdles forming lumpy solid masses. We can even add lemon extract to the hot milk to effect curdling immediately, but the taste and texture of the curd will not be the same as that of the curdling occurring in a few hours.
(ii) Fermentation: Fermentation is the process in which microorganisms such as yeast and certain bacteria break down sugar solution into alcohol and carbon-di-oxide. It is an irreversible process as the alcohol fosned cannot be turned back into sugar. Thus, fermentation is a chemical change.
Question 4.
Explain the characteristics of physical change with examples.
Answer:
(i) During a physical change, no new substances are formed. In a physical change,the chemical properties of a substance do not change. For example, when ice cube melts, water is formed. In this change, there is no new substance, but water is same both in ice and in water.
(ii) A physical change is usually temporary and reversible in nature. For example, when water is heated, water vapours are formed, once water vapours are cooled, water can be obtained again.
(iii) In a physical change, the chemical properties of a substance do not change. For example, when a piece of gold is melted, its chemical composition remains the same in the solid form and also in the liquid form.
(iv) In a physical change, the physical properties such as colour, shape and size of a substance may undergo a change.For example, cutting of vegetables and inflating a balloon are some examples of physical changes in which size and shape of a substance undergoes a change.
![]()
Question 5.
Write the characteristics of chemical change.
Answer:
- Heat, light or any other radiation may be given off or absorbed.
- Sound may be produced.
- A change in smell may take place (or) a new smell may be given off.
- A colour change may take place.
- A gas may be formed.
- Formation of precipitate
Question 6.
Explain about indicators of a chemical change with examples.
Answer:
(i) Take some broken pieces of egg shell in a test tube and add lemon juice to it. You could see bubbles of carbon-di-oxide evolving in the test tube. This is because of the chemical change between the two. Hence, we can say that evolution of bubbles serve as an indicator that of a chemical change.
(ii) When water is added to quicklime (calcium oxide) there will be evolution of lot of heat along with the formation of slaked lime (calciumhydroxide). This is a chemical change and it is indicated by the evolution of heat when the reaction sets in between quicklime and water.
(iii) Spoilage of food is a chemical change and it is indicated by the foul smell. So, change of odour is also an indicator of a chemical change.
(iv) When an iron nail is kept in water for a few days and taken out, the nail will become reddish brown in colour indicating that it has rusted. Rusting is a chemical change and it is indicated by a change in colour of the iron nail.
(v) A lump of curd is the precipitate that is obtained by the chemical reaction between hot milk and lemon juice. So, formation of precipitate is also an indication of a chemical change.
VII. Assertion – Reason type questions
Mark the correct choice as:
a. Both A and R are true and R is the correct explanation of A.
b. Both A and R are true but R is not the correct explanation of A.
c. A is true butR is false.
d. Both assertion and reason are false.
Question 1.
Assertion (A) : Burning of a candle is considered a physical as well as chemical change.
Reason (R)Melting of wax is a physical change melted wax turns into vapours and then bums which is a chemical change.
Answer:
(a) Both A and R are true and R is the correct explanation of A.
Question 2.
Assertion (A) : No-new substance is formed when water is heated to get steam.
Reason (R) :Conversion of water into steam is a physical change.
Answer:
(a) Both A and R are true and R is the correct explanation of A
Question 3.
Assertion (A) : Cutting of paper into very small pieces is an irreversible change.
Reason (R) : Physical changes are always reversible.
Answer:
(c) A is true but R is false
IX. Picture based Question:
Question 1.
Cut a fresh slice of potato, apple and brinjal and keep it away for sometime. What is the reason for the change of colour in these cases?

Answer:
- Colour of the potato and brinjal remains the same when stored in water but there is a change in colour with the piece kept in air.
- The slice of an apple acquires a brown colour if it is not consumed immediately.
- This occurs mainly due to oxidation which is caused by oxygen from the air.
- So, it is a chemical change.
Question 2.
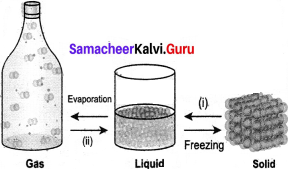
Answer:
- Meting
- Condensation
X. Higher order Thinking questions (HOTS):
Question 1.
Give an example to justify the statement that physical changes can be reversible as well as irreversible.
Answer:
- When water is frozen into ice, it is a physical change. It is reversible since ice can be converted to water again.
- When a piece of wood is cut into very small pieces, it is a physical change. It is reversible since small pieces of wood cannot be converted back to the bigger piece of wood.
- It shows that physical changes can be reversible as well as irreversible.
![]()
Question 2.
Photosynthesis is a chemical charge. Justify your answer.
Answer:
- Photosynthesis is the process through which plants convert light energy into chemical energy. Here is the chemical reaction involved.
- As we can see, water and carbon di oxide combine to form glucose and oxygen,
- Since new chemical species are formed, photosynthesis is clearly a chemical change.
Question 3.
Why is spoiling of food a chemical change?
Answer:
- Spoiling of food is a chemical change as it involves the breakdown of the food particles by the microbes.
- When the food gets spoiled certain properties of that food is loosed and some are gained, as there is a new change happening it is a chemical change.
- Spoilage of food is indicates by the foul-smell. So change of odour is an indicator of a chemical change.