Students can Download Chemistry Chapter 15 Chemistry in Everyday Life Questions and Answers, Notes Pdf, Samacheer Kalvi 12th Chemistry Book Solutions Guide Pdf helps you to revise the complete Tamilnadu State Board New Syllabus and score more marks in your examinations.
Tamilnadu Samacheer Kalvi 12th Chemistry Solutions Chapter 15 Chemistry in Everyday Life
Samacheer Kalvi 12th Chemistry Chapter 15 Chemistry in Everyday Life Textual Evaluation Solved
Samacheer Kalvi 12th Chemistry Chemistry in Everyday Life Multiple Choice Questions
Question 1.
Which of the following is an nalgesic?
(a) Streptornycin
(b) Chloromycetin
(c) Asprin
(d) Penicillin
Answer:
(c)Asprin
Question 2.
Dettol is the mixture of ……………
(a) Chioroxylenol and bithionol
(b) Chioroxylenol and a – terpineol
(c) phenol and iodine
(d) terpineol and bithionol
Answer:
(b) Chioroxylenol and a – terpineol
![]()
Question 3.
Antiseptics and disinfectants either kill or prevent growth of microorganisms. Identify which of the following statement is not true.
(a) dilute solutions of boric acid and hydrogen peroxide are strong antiseptics
(b) Disinfectants harm the living tissues
(c) A 0.2% solution of phenol is an antiseptic while 1% solution acts as a disinfectant
(d) Chlorine and iodine are used as strong disinfectants
Answer:
(a) dilute solutions of boric acid and hydrogen peroxide are strong antiseptics
Question 4.
Saccharin, an artificial sweetener is manufactured from ……………..
(a) cellulose
(b) toluene
(c) cyclohexene
(d) starch
Answer:
(b) toluene
Question 5.
Drugs that bind to the receptor site and inhibit its natural function are called …………….
(a) antagonists
(b) agonists
(c) enzymes
(d) molecular targets
Answer:
(a) antagonists
Question 6.
Aspirin is a/an ……………..
(a) acetylsalicylic acid
(b) benzoyl salicylic acid
(c) chlorobenzoic acid
(d) anthranilic acid
Answer:
(a) acetylsalicylic acid
Question 7.
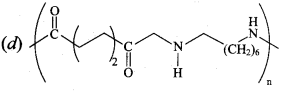
Which one of the following structures represents nylon 6,6 polymer?
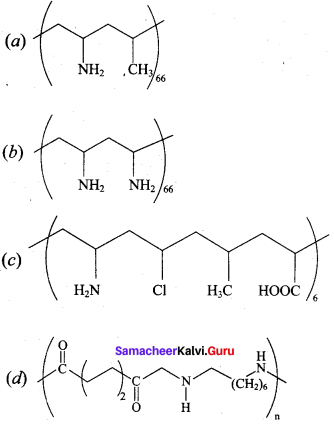
Answer:
Question 8.
Natural rubber has ………..
(a) alternate cis – and trans – configuration
(b) random cis – and trans-configuration
(c) all cis – configuration
(d) all trans – configuration
Answer:
(c) all cis – conflguration
Question 9.
Nylon is an example of …………..
(a) polyamide
(b) polythene
(c) polyester
(d) poly saccharide
Answer:
(a) polyamide
![]()
Question 10.
Terylene is an example of …………..
(a) polyamide
(b) polythene
(c) polyester
(d) poly saccharide
Answer:
(c) polyester
Question 11.
Which is the monomer of neoprene in the following?
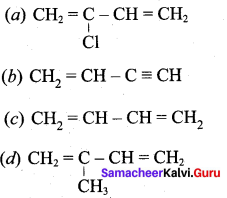
Answer:
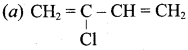
Question 12.
Which one of the following is a bio-degradable polymer?
(a) HDPE
(b) PVC
(c) Nylon 6
(d) PHBV
Answer:
(d) PHBV
Question 13.
Non stick cook wares generally have a coating of a polymer, whose monomer is ………….
(a) ethane
(b) prop – 2 – enenitrile
(c) chioroethene
(d) 1, 1, 2, 2 – tetrafluoroethane
Answer:
(d) 1, 1, 2, 2 – tetrafluoroethane
Question 14.
Assertion: 2 – methyl – I ,3 – butadiene is the monomer of natural rubber
Reason: Natural rubber is formed through aniònic addition polymerisation.
(a) If both assertion and reason are true and reason is the correct explanation of assertion
(b) if both assertion and reason are true but reason is not the correct explanation of assertion
(c) assertion is true but reason is false
(d) both assertion and reason are false
Answer:
(c) assertion is true but reason is false
![]()
Question 15.
An example of antifertility drug is ………….
(a) novesirol
(b) seldane
(c) salvarsan
(d) Chioramphenicol
Answer:
(a) novestrol
Question 16.
The drug used to induce sleep is …………..
(a) paracetamol
(b) bithional
(c) chioroquine
(d) equanil
Answer:
(d) equanil
Question 17.
Which of the following is a co – polymer?
(a) Orlon
(b) PVC
(c) Teflon
(d) PHBV
Answer:
(d) PHBV
Question 18.
The polymer used in making blankets (artificial wool) is ……………
(a) polystyrene
(b) PAN
(c) polyester
(d) polythene
Answer:
(b) PAN
![]()
Question 19.
Regarding cross-linked or network polymers, which of the following statement is incorrect?
(a) Examples are Bakelite and melamine
(b) They are formed from bi and tri-functional monomers
(c) They contain covalent bonds between various linear polymer chains
(d) They contain strong covalent bonds in their polymer chain
Answer:
(d) They contain strong covalent bonds in their polymer chain
Question 20.
A mixture of chioroxylenol and terpinecol acts as ……………
(a) antiseptic
(b) antipyretic
(c) antibiotic
(d) analgesic
Answer:
(a) antiseptic
II. Answer the following questions
Question 1.
Which chemical is responsible for the antiseptic properties of dettol?
Answer:
- Two main constituents of dettol is chloroxylenol and terpineol.
- But among these two chloroxylenol plays an important role as an antiseptic.
Question 2.
What are antibiotics?
Answer:
Antibiotics is a chemical substance produced by one microorganism, that selectively inhibits the growth of another micro organism. Example : penicillins and cephalosporins.
Question 3.
Name one substance which can act as both analgesic and antipyretic.
Answer:
Aspirin can act as both analgesic and antipyretic,
![]()
Question 4.
Write a note on synthetic detergents.
Answer:
1. Synthetic detergents are formulated products containing either sodium salts of alkyl hydrogen suiphates or sodium salts of long chain alkyL benzene suiphonic acids.
2. Synthetic detergents are three types. They are
- anionic detergents – sodium lauryl sulphate.
- Cationic detergents – n – hexadecyltrimethyl ammonium chloride.
- Non-ionic detergents – Pentaerythrityl stearate.
3. Synthetic detergents can be used even in hard water, while soaps cannot be used in hard water.
4. The cleansing action of detergents are similar to the cleansing action of soaps.
5. When detergents are dissolved in water its hydrocarbon part attaches itself to grease and oil particles. Whereas its ionic part remains attached to water. Therefore when dirty clothes are agitated in solution of detergents then dirty particles sticks to the hydrocarbon part of detergents and at the same time the water loving ionic part pulls away this dirt from clothes.
Question 5.
How do antiseptics differ from disinfectants?
Answer:
Antiseptics
- Antiseptics are chemical substance which prevent the growth of micro organizers and may even kill them but are not harmful to living tissues.
- They are generally applied to living tissues such as wounds, cuts bulks and diseased surfaces.
- All the antiseptics are disinfectants.
- They are not ingested or swallowed.
- e.g., Povidone – iodine, Benzalkonium – Chloride
Disinfectants
- Disinfectants are chemical substances which kill microorganism or stop their growth but are harmful to human tissues.
- Disinfectants are applied to inanimated objects such as floors, drainage system, instruments etc.
- All the disinfectants are not antiseptics.
- They can be injected or swallowed.
- e.g. Alcohol, chlorine compunds.
![]()
Question 6.
What are food preservatives?
Answer:
- Food preservatives are substances capable of inhibiting, retarding or arresting the process of fermentation, acidification or other decomposition of food by growth of microorganisms.
- Ex.: Acetic acid, Sodium metasulphite, Sodium benzoate.
Question 7.
Why do soaps not work ¡n hard water?
Answer:
Soaps are sodium or potassium salts of long – chain falty acids. Hard water contains calcium and magnesium ions. When soaps are dissolved in hard water, these ions displace sodium or potassium from insoluble calcium or magnesium salts of fatty acids. These insoluble salts separate as scum.
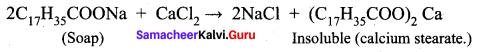
This is the reason why soaps do not work in hard water.
![]()
Question 8.
What are drugs? How are they classified?
Answer:
- A drug is a substance that is used to modify or explore physiological systems or pathological states for the benefit of the recipient.
- It is used for the purpose of diagnosis, prevention cure/relief of disease.
Drugs are classified based on their properties such as
- Chemical structure
- Pharmacological effect
- Target system
- Site of action
Question 9.
How do the tranquilizers work in the body?
Answer:
- They are neurologically active drugs.
- Tranquilizer acts on the central nervous system by blocking the neurotransmitter dopamine in the brain.
- This drug is used for treatment of stress anxiety, depression, sleep disorders and severe mental diseases like schizophrenia.
![]()
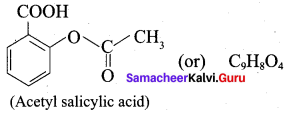
Question 10.
Write the structural formula of aspirin.
Answer:
Question 11.
Explain the mechanism of cleansing action of soaps and detergents.
Answer:
Mechanism of cleansing action of soaps and detergents:
1. The cleansing action of both soaps and detergents from their ability to lower the surface tension of water, to emulsify oil or grease and to hold them in a suspension in water.
2. This ability is due to the structure of soaps and detergents.
3. In water a sodium soap dissolves to form soap anions and sodium cations. For example, the following chemical equation shows the ionisation of sodium palmitate.
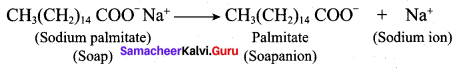
4. A soap anion consists of a long hydrocarbon chain with a carboxylate group on one end. The hydrocarbon chain, which is hydrophobic, is soluble in oils or grease. The ionic part is the carboxylate group which is hydrophilic, is soluble in water.
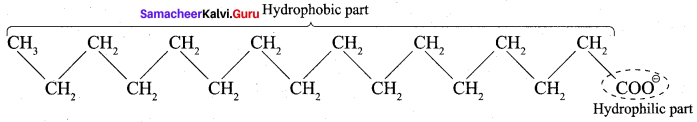
5. In water, detergent dissolves to form detergent anions and sodium cations. For example the following chemical equations show the ionisation of sodium alkyl sulphate and sodium alkyl benzene sulphate.
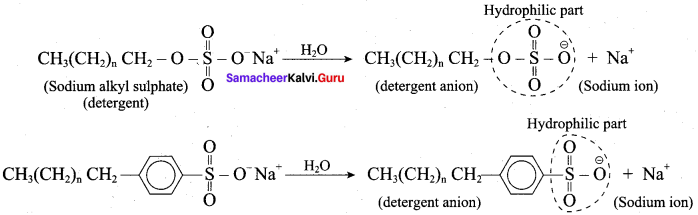
6. The following explains the cleansing action of a soap or detergent on a piece of cloth with a greasy stain.
- A soap or detergent anion consists of a hydrophobic part and a hydrophilic part.
- Soap or detergent reduces the surface tension of water. Therefore the surface of the cloth is wetted thoroughly.
- The hydrophobic parts of the soap or detergents anions are soluble in grease.
- The hydrophilic parts of the anions are soluble in water.
- Scrubbing or mechanical agitation helps to pull the grease away from the cloth and the grease is broken into smaller droplets.
- Repulsion between the droplets causes the droplets to be suspended in water, fonning an emulsion.
- Thus the droplets do not coagulate or,redeposit on the cloth. Rinsing washes away the droplets.
![]()
Question 12.
Which sweetening agent are used to prepare sweets for a diabetic patient?
Answer:
Sweetening agent used to prepare sweets for a diabetic patient are Saccharin, Aspartame, alitame etc…
Question 13.
What are narcotic and non – narcotic drugs. Give examples.
Answer:
1. Narcotic drug is an addictive drug that reduces pain, induces sleep and may alter mood or behaviour. Example: Morphine and codeine.
2. Non – narcotic drug are chemical substance (medications) used to control pain and inflammation. They are available at drugstores without a prescription or by prescription when given at higher doses. Example: Acetaminophen and paracetamol.
Question 14.
What are anti-fertility drugs? Give examples.
Answer:
Anti-fertility drugs are synthetic hormones that suppress ovulation/fertilization.
Ex.: Synthetic oestrogen – i) Ethynylestradiol ii) Menstranol
Synthetic Progesterone – i) Norethindrone ii) Norethynodrel
Question 15.
Write a note on copolymer.
Answer:
- A polymer containing two or more different kinds of monomer units is called a co-polymer.
- Co – polymers have properties quite different from the homopolymers.
- The structural units of co-polymers are derived from the different monomers may be present in regular, alternation or in random order or strings of several units of one kind may alternate with strings of another.
- For example, Buna – S, Buna – N, Nylon – 6,6 etc. Buna – S contains styrene and butadiene monomer units.
![]()
Question 16.
What are biodegradable polymers? Give examples.
Answer:
- Natural polymers which degrade on their own or by microorganisms after a certain period of time are called biodegradable polymers.
- Ex.: Poly hydroxybutyrate (PHB), Polyglycolic acid (PGA), Polylactic acid (PLA)
Question 17.
How is terylene prepared?
Answer:
The monomers are ethylene glycol and terephthalic acid or dimethýlterephthalate. When these monomers are mixed and heated at 500K in the presence of zinc acetate and antimony trioxide catalyst, terylene (or dacron) is formed.
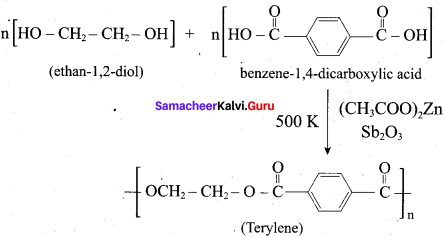
Question 18.
Write a note on the vulcanization of rubber.
Answer:
- When natural rubber is heated with sulphur it becomes strong and elastic. This process is known as vulcanization of rubber.
- Natural rubber is mixed with 3-5% sulphur and heated at 100-150°C.
- This causes the cross-linking of the cis-1,4- polyisopre’ne chains through disulphide (-S-S-) bonds.
- The physical properties of rubber can be altered by controlling the amount of sulphur that is used for vulcanization.
- If 1 – 3% sulphur is added the rubber is soft and stretchy.
- If 3 -10% sulphur is added the rubber is somewhat harder but flexible.
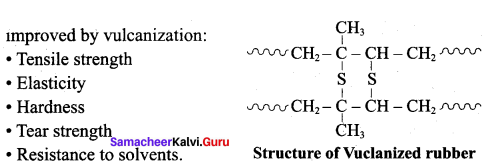
Question 19.
Classify the following as linear, branched or cross-linked polymers …………………..
- Bakelite
- Nylon
- polythene
Answer:
- Bakelite – cross-linked polymer
- Nylon – Linear polymer
- Polythene – Linear polymer
Question 20.
Differentiate thermoplastic and thermosetting.
Answer:
Difference between thermoplastic and thermosetting:
Thermoplastic
- They soften on heating and harden on cooling, and they can be remoulded.
- They consists of linear long çhain polymers and low molecular weights polymers.
- All the polymer chains are held together by weak Van der Waals forces.
- They are weak, soft and less brittle.
- They are formed by adding a polymerisation
- They are soluble in organic solvents.
- Example: PVC, polythene, polystrene etc.
Thermosetting
- They do not soften on heating and they cannot be remoulded.
- They consist of a three-dimensional network structure and high molecular weight polymers.
- All the polymer chains are linked by strong covalent.
- They are strong, hard and more brittle.
- They are formed by condensation polymerisation.
- They are insoluble in organic solvents.
- Example: Bakelite, melamine etc.
Samacheer Kalvi 12th Chemistry Chemistry in Everyday Life Additional Questions
Samacheer Kalvi 12th Chemistry Chemistry in Everyday Life 1 mark Questions and Answers
I. Choose the best answer.
Question 1.
The substance that is used to modify the physiological system for the benefit of the recipient is called …………….
(a) a drug
(b) a dye
(c) a food preservative
(d) soap
Answer:
(a) a drug
![]()
Question 2.
Which one interacts with macromolecular targets such as proteins to produce a therapeutic and useful biological response?
(a) Detergent
(b) cleansing agent
(c) medicine
(d) food preservative
Answer:
(c) medicine
Question 3.
The ratio between the maximum tolerated dose of a drug and a minimum curative dose is called …………..
(a) iso electric point
(b) therapeutic index
(c) critical point
(d) iso thermal point
Answer:
(b) therapeutic index
Question 4.
Which one of the following does not belong to penicillin group?
(a) Ampicillin
(b) Amoxicillin
(c) catecholamine
(d) mithicillin
Answer:
(c) catecholamine
Question 5.
Which of the following does belongs to penicillin group drugs?
(a) Mithicillin
(b) opiates
(c) steroids
(d) catecholamine
Answer:
(a) Mithicillin
![]()
Question 6.
Which one of the following is an antibiotic?
(a) erythromycin
(b) atenolol
(c) amlodipine
(d) propranolol
Answer:
(a) erythromycin
Question 7.
Which one of the following is not an antibiotic?
(a) amoxicillin
(b) cefixime
(c) amlodipine
(d) ampiciflin
Answer:
(c) amlodipine
Question 8.
Which one of the following is an example tbr antihypertensive drug?
(a) atenolol
(b) amoxicillin
(c) cefixime
(d) tetracycline
Answer:
(a) atenolol
Question 9.
Which of the following does not belongs to antihypertensive drug?
(a) atenolol
(b) amlodipine
(c) propranolol
(d) erythromycin
Answer:
(d) erythromycin
Question 10.
Which one of the following inhibits the initiation of protein synthesis?
(a) streptomycin
(b) erythromycin
(c) atenolol
(d) amlodipine
Answer:
(a) streptomycin
Question 11.
Which one of the following prevents the incorporation of new amino acids to the protein?
(a) atenolol
(b) streptomycin
(c) erythromycin
(d) tetracycline
Answer:
(c) erythromycin
Question 12.
Which one of the following inhibits the bacterial growth?
(a) p – amino benzoic acid
(b) sulphanilamide
(c) folic cid
(d) sodium benzoate
Answer:
(b) sulphanilamide
![]()
Question 13.
Which of the following is needed by many bacteria to produce folic acid?
(a) PABA
(b) DHPS
(c) TNB
(d) GTN
Answer:
(a) PABA
Question 14.
Which of the following is called PABA?
Answer:
(a) p – nitro benzanilic acid
(b) p – amino butyric acid
(c) p – amino benzene suiphonic acid
(d) p – amido benzene suiphonyl chloride
Answer:
(c) p – amino benzene suiphonic acid
Question 15.
Which one of the following binds to the receptor site should inhibit its natural function?
(a) antacids
(b) antioxidant
(c) antibiotics
(d) antagonists
Answer:
(d) antagonists
Question 16.
Which of the following is used in the reduced sleepiness?
(a) caffeine
(b) morphine
(c) suiphanilide
(d) p – aminobenzene sulphonic acid
Answer:
(a) caffeine
Question 17.
Which one of the following is used as painkiller?
(a) lodoform
(b) chloropicrin
(c) morphine
(d) coffeine
Answer:
(c) morphine
Question 18.
Which of the following is not an example of antacid?
(a) Histamine
(b) cimetidine
(c) ranitidine
(d) erythromycin
Answer:
(d) erythromycin
Question 19.
Which one of the following is used as an antacid?
(a) magnesium hydroxide
(b) aluminium hydroxide
(c) ranitidine
(d) all the above
Answer:
(d) all the above
![]()
Question 20.
Which one of the following is used to treat stress, anxiety, depression, sleep disorder and schizopherenia?
(a) Tranquilizer
(b) antibiotic
(c) analgesic
(d) opioids
Answer:
(a) Tranquilizer
Question 21.
Which one of the following is an example for tranquilizer?
(a) cimetidine
(b) diazepam
(c) histamine
(d) PABA
Answer:
(b) diazepam
Question 22.
Identify the medine that is used to treat stress, anxiety. depression and schizophrenia.
(a) valium
(b) cimetidinc
(c) chiorofom
(d) adenosine
Answer:
(a) valium
Question 23.
Which one of the following is used to reduce fever and prevent platelet coagulation?
(a) antibiotic
(b) antiseptic
(c) antioxidant
(d) antipyretic
Answer:
(d) antipyretic
Question 24.
Which one of the following is an anti inflamatory drug?
(a) morphine
(b) coheinc
(c) aspirin
(d) histidine
Answer:
(c) aspirin
Question 25.
Which one of the following is used to cure headache, muscle strain, arthritis?
(a) acetaminophen
(b) ibuprofen
(c) aspirin
(d) all the above
Answer:
(d) all the above
Question 26.
Which one of the foLlowing is used in the prevention of heart attacks?
(a) aspirin
(b) ibuprofen
(c) paracetamol
(d) morphine
Answer:
(a) aspirin
Question 27.
Which one of the following is an example of an àntipyretic?
(a) acetyl salicylic acid
(b) methyl salicylate
(c) paraldehyde
(d) diethyl ether
Answer:
(a) acetyl salicylic acid
Question 28.
Which one of the following is a non steroidal anti inflammatory drug?
(a) aspirin
(b) morphine
(c) haloperidol
(d) ibuprofen
Answer:
(d) ibuprofen
Question 29.
Which of the following is a major tranquilizer?
(a) diazepam
(b) valium
(c) clozapine
(d) alprazolm
Answer:
(c) clozapine
Question 30.
Which of the following is a minor tranquilizer?
(a) haloperidol
(b) clozapine
(c) morphine
(d) valium
Answer:
(d) valium
![]()
Question 31.
Consider the following statements
(i) Tranquilizers act on the central nervous system by blocking the neurotransmitter dopamine in the brain.
(ii) Histamines stimulate the secretion of HCI by activating the receptor in the stomach wall.
(iii) The antibiotic cimetidine inhibits the bacterial growth.
Which of the above statement is/are not correct?
(a) (i) only
(b) (¡) & (ii)
(c) (iii) only
(d) (ii) only
Answer:
(c) (iii) only
Question 32.
Consider the following statements
(i) Acetaminophen reduces fever by causing the hypothalamus to override a prostaglandin
(ii) opioids relieve pain and produce sleep and are addictive
(iii) Aspirin finds useful in the pain of terminal cancer.
Which of the above statement is/arc not correct?
(a) (i) only
(b) (ii) only
(c) (ii) & (iii)
(d) (iii) only
Answer:
(d) (iii) only
Question 33.
Which of the following are addictive and poisonous drug?
(a) ibuprofen
(b) aspirin
(c) morphine
(d) paracetamol
Answer:
(c) morphine
Question 34.
Which of the following are used for post operative pain and pain of terminal cancer?
(a) morphine, codeine
(b) ibuprofen, aspirin
(c) methyl salicylate, salicylic acid
(d) histidine, ranitidine
Answer:
(a) morphine, codeine
Question 35.
Which one of the following is an local anaesthetic?
(a) lidocaine
(b) Propofol
(c) iso flurane
(d) ibuprofen
Answer:
(a) lidocaine
![]()
Question 36.
Which one of the following is an example of general anaesthetic?
(a) propofol
(b) isoflurane
(c) ranitidine
(d) omeprazole
Answer:
(b) isoflurane
Question 37.
Identify the intraveneous general anaesthetics?
(a) milk of magnesia
(b) lidocaine
(c) omeprazole
(d) iso fharane
Answer:
(d) iso fharane
Question 38.
Which one of the following is an inhalational general anaesthetic?
(a) procain
(b) iso fiurane
(c) lidocaine
(d) rabeprazole
Answer:
(b) iso fiurane
Question 39.
Which one of the following is an antacid?
(a) omeprazole
(b) rabeprazole
(c) milk of magnesia
(d) all the above
Answer:
(d) all the above
![]()
Question 40.
Consider the following statements.
(i) Propofol cause a controlled and reversible loss of consciousness by affecting central nervous system.
(ii) Ibuprofen is used for major surgical procedures.
(iii) Lidocaine is used to relieve burning sensation in the chest / throat area.
Which of the above statement is/are not correct?
(a) (i) only
(b) (i) & (ii)
(c) (ii) & (iii)
(d) (i) & (iii)
Answer:
(c) (ii) & (iii)
Question 41.
Which one of the following is not an antacid?
(a) propofol
(b) ranitidine
(c) omeprazole
(d) rabeprazole
Answer:
(a) propofol
Question 42.
Which one of the following is used to provide relief from the allergic effects?
(a) cetrizine
(b) ampicillin
(c) erythromycin
(d) milk of magnesia
Answer:
(a) cetrizine
Question 43.
Which one of the following inhibits bacterial cell wall biosynthesis?
(a) eryLhromycin
(b) azithromycin
(c) penicillin
(d) cetrizine
Answer:
(c) penicillin
Question 44.
Which of the following is used to treat respiratory tract infections, genital, gastrointestinal tract and skin infections?
(a) ampicillin
(b) penicillin
(c) terfenadine
(d) azithromycin
Answer:
(d) azithromycin
![]()
Question 45.
Which one of the following is used to treat urinary tract infection and respiratory infections?
(a) doxycycline
(b) karamycin
(c) ciprolloxacin
(d) ibuprofen
Answer:
(c) ciprolloxacin
Question 46.
Which of the following is used in the treatment of cholera, acne vulgaris?
(a) fluoro quinolone
(b) aminoglycosides
(c) tetracycline
(d) macrolides
Answer:
(c) tetracycline
Question 47.
Which one of the following is used to treat infections caused by gram negative bacteria?
(a) kanamycin
(b) gentamycin
(c) neomycin
(d) all the above
Answer:
(d) all the above
Question 48.
Which one of the following inhibits bacterial enzyme DNA gyrase?
(a) doxy cycline
(b) kanamycin
(c) ciprofloxacin
(d) aspirin
Answer:
(c) ciprofloxacin
Question 49.
Which one of the following is an antiseptic?
(a) Hydrogen peroxide
(b) alcohol
(c) menstranol
(d) chlorine compounds
Answer:
(a) Hydrogen peroxide
![]()
Question 50.
Which one of the following is used to reduce the risk of infection during surgery?
(a) povidone – iodine
(b) ethynyles tradiol
(c) norethindrone
(d) acetyl salicylic acid
Answer:
(a) povidone – iodine
Question 51.
Consider the following statements
(i) Oestrogen, menstranol are synthetic hormones that suppresses ovulation / fertilisation,
(ii) Norethindrone used in birth control pills.
(iii) Chlorine compounds are used to reduce the risk of infection during surgery.
Which of the above statement is/are not correct?
(a) (i) only
(b) (ii) & (iii)
(c) (iii) only
(d) (i) & (iii)
Answer:
(c) (iii) only
Question 52.
Which one of the following is used as a preservative for the preparation of pickles and preservation of vegetables?
(a) sodium acetate
(b) acetic acid
(c) sodium carbonate
(d) salicylic acid
Answer:
(b) acetic acid
Question 53.
Which one is used as preservatives for fresh vegetables and fruits?
(a) Palmitic acid
(b) Palm oil
(c) sodium meta suiphite
(d) sulphur dioxide
Answer:
(c) sodium meta suiphite
Question 54.
Which one of the following is used as an emulsifier?
(a) sodium meta suiphite
(b) sucrose ester of palmiticacid
(c) sodium benzoate
(d) sodium bi carbonate
Answer:
(b) sucrose ester of palmiticacid
![]()
Question 55.
Which method is used to preserve food?
(a) pasteurisation & irration
(b) chilling and freezing
(c) drying and dehydration
(d) all the above
Answer:
(d) all the above
Question 56.
Which one of the following act as an antioxidant?
(a) Palmîtic acid
(b) butyl hydroxy toluene
(c) sodium benzoate
(d) Ascorbic acid
Answer:
(b) butyl hydroxy toluene
Question 57.
Consider the following statements.
(i) Addition of vitamins and minerals reduces the mall nutrient.
(ii) Flouring agents reduces the aroma of the food.
(iii) Antioxidants produce the formation of potentially toxic oxidation products of lipids.
Which of the above statement is/are not correct?
(a) (i) only
(b) (ii) only
(c) (ii) & (iii)
(d) (i) & (iii)
Answer:
(c) (ii) & (iii)
Question 58.
Which of the following is not a sugar substituent?
(a) Sorbitol
(b) mannitol
(c) xylitol
(d) cresol
Answer:
(d) cresol
Question 59.
Which of the following is not a sugar substituent?
(a) Butyl hydroxy toluene
(b) Butylated hydroxy anisole
(c) Aspartame
(d) Ascorbic acid
Answer:
(c) Aspartame
![]()
Question 60.
Identify the artificial sweeteners.
(a) Saccharin, sucralose
(b) culutaric acid, glycollic acid
(c) BHT, BHA
(d) GTN, TNG
Answer:
(a) Saccharin, sucralose
Question 61.
Glyceryl ester of long chain fatty acids are called …………..
(a) soap
(b) detergent
(c) antiseptic
(d) antibiotic
Answer:
(a) soap
Question 62.
Which one of the following describes the quality of soap?
(a) TFT value
(b) TFM value
(c) PPM value
(d) TFP value
Answer:
(b) TFM value
Question 63.
Sodium salt of long chain allyl benzene sulphomc acids are called ……………….
(a) soap
(b) detergent
(c) disinfectant
(d) antiseptic
Answer:
(b) detergent
Question 64.
Which one of the following is an anionic detergent?
(a) n – hexa decyl tri methyl ammonium chloride
(b) Peifla erythntyl stearate
(c) Sodium lauryl sulphate
(d) 3 – hydroxy – 2, 2 bis (hydroxy methyl) propyl heptonoate
Answer:
(c) Sodium lauryl sulphate
Question 65.
Which of the following is an example of catìonic detergent?
(a) Sodium lauryl sulphate
(b) sodium pahnitate
(c) sodium dodecyl benzene suiphonate
(d) n – hexa decyl timethyl ammonium chloride
Answer:
(d) n – hexa decyl timethyl ammonium chloride
Question 66.
Which one of the following is an example of non-ionic detergent?
(a) sodium lauryl sulphate
(b) n – hexa decyl trimethyl ammonium chloride
(c) Penta erythrityl stearate
(d) N, N, N – trimethyl hexa decan – 1 – aminium chloride
Answer:
(c) Penta erythrityl stearate
Question 67.
Which one of the following is a natural polymer?
(a) cellulose, silk
(b) PVC, Polythene
(c) Buna – N, Buna – S
(d) Bakelite, Nylon 6,6
Answer:
(a) cellulose, silk
![]()
Question 68.
Which one of the following is a synthetic rubber?
(a) Neoprene
(b) cellulose
(c) silk
(d) poly isoprene
Answer:
(a) Neoprene
Question 69.
Which one of the following is a semisynthetic polymer?
(a) poly isoprene
(b) viscose rayon
(c) nylon
(d) terylene
Answer:
(b) viscose rayon
Question 70.
Which one of the following is not a cross linked polymer?
(a) poly propylene
(b) bakelite
(c) melamine
(d) urea formaldehyde
Answer:
(a) poly propylene
Question 71.
Identify the thermo setting plastic?
(a) nylon 6, 6
(b) neoprene
(c) melamine
(d) bakelite
Answer:
(c) melamine
Question 72.
Which of the following is a thermoplastic?
(a) bakelite
(b) melamine
(c) urea formaldehyde
(d) polystrene
Answer:
(d) polystrene
![]()
Question 73.
Which one of the following is an elastomer?
(a) nylon 6,6
(b) terylene
(c) buna – S
(d) bakelite
Answer:
(c) buna – S
Question 74.
Which one of the following is an example for addition polymer?
(a) polyethylene
(b) PVC
(c) teflon
(d) all the above
Answer:
(d) all the above
Question 75.
Which one of the following is an example of condensation polymer?
(a) poly ethylene
(b) polyester
(c) PVC
(d) teflon
Answer:
(b) polyester
Question 76.
Which one of the following is not an additional polymer?
(a) poly ethylene
(b) PVC
(c) Nylon 66
(d) teflon
Answer:
(c) Nylon 66
Question 77.
Consider the following statements
(i) Nylon-6, 6 are polymerchains form fibres by hydrogen bonding.
(ii) Thermoplastic become hard on heating and soft on cooling and cannot be remoulded.
(iii) Cellulose and silk are synthetic polymers.
Which of the above statement is/are not correct? ,
(a) (i) only
(b) (ii) & (iii)
(c) (iii) only
(d) (i) & (iii)
Answer:
(b) (ii) & (iii)
![]()
Question 78.
Which one of the following is used as an free radical initiator in the preparation of polystrene?
(a) hydrogen peroxide
(b) methyl chloride
(c) Benzoyl peroxide
(d) Benzyl peroxide
Answer:
(c) Benzoyl peroxide
Question 79.
Which mechanism is followed in the synthesis of polystrene?
(a) free radical polymerisation
(b) cationic polymensation
(c) Anionic polymerisation
(d) SN1 mechanism
Question 80.
Which one of the polymer is used as insulation for cables, making toys?
(a) HDPE
(b) LDPE
(c) teflon
(d) orlon
Answer:
(b) LDPE
Question 81.
Which one of the following catalyst is used in the preparation of high density polyethylene?
(a) benzoyi peroxide
(b) zeigler natta catalyst
(c) ammonium per sulphate
(d) hydrogen peroxide
Answer:
(b) zeigler natta catalyst
Question 82.
Identify the zeiglar natta catalyst.
(a) TiCI4 + (C2H5)3AI
(b) (C2H5)4Pb + TiCl4
(c) AICl3 + HCI
(d) ZnCI2 + Cone. HCI
Answer:
(a) TiCI4 + (C2H5)3AI
![]()
Question 83.
Which of the following is used to make bottles and pipes?
(a) LDPE
(b) Terylene
(c) PVC
(d) HDPE
Answer:
(a) LDPE
Question 84.
Which polymer is used in preparing non-sticking utensils?
(a) orlon
(b) PAN
(c) teflon
(d) HDPE
Answer:
(c) teflon
Question 85.
Which one of the following is used as a substitute of wool for making blankets, sweaters?
(a) orlon
(b) terylene
(c) polyester
(d) nylon
Answer:
(a) orlon
Question 86.
What are the raw materials required for the manufacture of Nylon 6, 6?
(a) caprolactam + hydrazine
(b) adipic acid + hexa methylene diamine
(c) methanal + ammonia
(d) phenol + methanal
Answer:
(b) adipic acid + hexa methylene diamine
Question 87.
Which one of the following is not a condensation polymer?
(a) nylon 6, 6
(b) nylon 6
(c) polyethylene
(d) terylene
Answer:
(c) polyethylene
![]()
Question 88.
Which one is used in the manufacture of nylon-6?
(a) adipic acid + hexamethylene diamine
(b) succinic acid + hexamethylene tetramine
(c) ∈-amino carproic acid
(d) adipic acid + hexamethylene tetramine
Answer:
(c) ∈-amino carproic acid
Question 89.
Which one of the following is the other name of nylon 6, 6?
(a) poly urethane
(b) urotropine
(c) poly caprolactum
(d) poly hexametheylene adipamide
Answer:
(d) poly hexametheylene adipamide
Question 90.
Which one of the following is used in the manufacture of tyrecards fabrics?
(a) nylon 6, 6
(b) nylon 6
(c) orlon
(d) dacron
Answer:
(b) nylon 6
Question 91.
What are the raw materials required for the manufacture of terylene?
(a) ethylene glycol + terephthalic acid
(b) phthalic auhydride + phenol
(c) adipic acid + hexamethylene diamine
(d) phenol + methanal
Answer:
(a) ethylene glycol + terephthalic acid
![]()
Question 92.
Name the catalyst used in the preparation of terylene?
(a) zeiglar natta catalyst
(b) zincacetate + antimony oxide
(c) benzoyi peroxide
(d) ammonium persuiphate
Answer:
(b) zincacetate + antimony oxide
Question 93.
Which one of the following is used as glass reinforcing material in safety helmets?
(a) nylon
(b) bakelite
(c) terylene
(d) orlon
Answer:
(c) terylene
Question 94.
What are the raw materials required for the manufacture of bakelite?
(a) ethane 1, 2 – diol + benzene 1, 4 – dicarboxylic acid
(b) phenol + methanal
(c) adipic acid + hexamethylene diamine
(d) isoprene + methanal
Answer:
(b) phenol + methanal
Question 95.
Linear polymer of phenol formal dehyde is called
(a) novolac
(b) bakelite
(c) terylene
(d) orlon
Answer:
(a) novolac
Question 96.
Which one of the following is used to prepare combs and pens?
(a) navolac
(b) soft bakelite
(c) hard bakelite
(d) neoprene
Answer:
(a) navolac
![]()
Question 97.
Which one of the following thermo setting plastic is used in paints?
(a) melamine
(b) hard bakelite
(c) navolac
(d) soft bakelite
Answer:
(c) navolac
Question 98.
Which one of the following is used for making unbreakable crockery?
(a) phenol formal dehyde
(b) melamine formal dehyde
(c) urea formal dehyde
(d) navolac
Answer:
(b) melamine formal dehyde
Question 99.
What are the raw materials required to prepare Buna – S rubber?
(a) phenol + methanal
(b) melamine + methanal
(c) styrene + butadiene
(d) adipic acid + methanal
Answer:
(c) styrene + butadiene
Question 100.
Which one of the following element is used in vulcanization of rubber?
(a) oxygen
(b) nitrogen
(c) carbon
(d) sulphur
Answer:
(d) sulphur
Question 101.
Which one of the following is a natural rubber?
(a) Buna-S
(b) Buna-N
(c) cis – 1, 4 – poly isoprene
(d) neoprene
Answer:
(c) cis – 1, 4 – poly isoprene
Question 102.
The raw material is used in the manufacture of ieoprene?
(a) isoprene
(b) chloroprene
(c) 1, 3 – buta diene
(d) vinyl chloride
Answer:
(b) chloroprene
![]()
Question 103.
Which one of the following rubber is used in the manufacture of chemical container and conveyer belts?
(a) Buna – N
(b) neo prene
(c) Buna – S
(d) poly isoprene
Answer:
(b) neo prene
Question 104.
The raw materials required for the manufacture of Buna – N are …………..
(a) acrylonitrile + Buta – 1, 3 – diene
(b) chloro prene + buta – 1, 3 – diene
(c) terephthalic acid + ethane 1, 2 – diol
(d) phenol + methanal
Answer:
(a) acrylonitrile + Buta – 1, 3 – diene
Question 105.
Which of the following are required to prepare Buna – S?
(a) vinyl cyanide + 1, 3 – butadiene
(b) chioro prene + buta -1, 3 – diene
(c) buta – 1, 3 – diene + styrene
(d) isoprene + styrene
Answer:
(c) buta – 1, 3 – diene + styrene
Question 106.
Which of the following used in medical field such as surgical sutures, 1asma substitute?
(a) PHBV
(b) PLA
(c) PCE
(d) all the above
Answer:
(d) all the above
Question 107.
Which one of the following is not an example of biodegradable plastic?
(a) polyhydroxy butyrate
(b) poly glycollic acid
(c) polythene
(d) poly caprolactone
Answer:
(c) polythene
![]()
Question 108.
Which of the following is an example for bio degradable plastic?
(a) polystyrene
(b) poly vinyl chloride
(c) bakelite
(d) polylactic acid
Answer:
(d) polylactic acid
Question 109.
Which one of the following is used in orthopaedic devices and in controlled release of drugs?
(a) PHB
(b) PHBV
(c) PGA
(d) PLA
Answer:
(b) PHBV
Question 110.
Glycine and e-amino caproic acid polymenses to give …………..
(a) glycyl amine
(b) nylon 6, 6
(c) Nylon – 2 Nylon 6
(d) orlon
Answer:
(c) Nylon – 2 Nylon 6
Question 111.
Which one of the following is used in making automobiles and foot wear?
(a) Bun – S
(b) Buna – N
(c) natural rubber
(d) neoprene
Answer:
(a) Bun – S
Question 112.
Which one of the following is used as an insulator and making conveyor belts?
(a) terylene
(b) orlon
(c) neoprene
(d) Buna – N
Answer:
(c) neoprene
![]()
Question 113.
Which type of nylon is used in making brushes, synthetic fibres, parachute, ropes and carpets?
(a) nylon – 2
(b) nylon – 6
(c) nylon 6,6
(d) nylon – 2, nylon 6
Answer:
(c) nylon 6,6
Question 114.
Which one is used in making non-breakable cups and laminated sheets?
(a) bakelite
(b) urea formaldehyde
(c) PHBV
(d) teflon
Answer:
(b) urea formaldehyde
Question 115.
Which of the polymer is used in making fibres, safety belts, lyre cords and ropes?
(a) terylene
(b) orlon
(c) Nylon
(d) bakelite
Answer:
(a) terylene
Question 116.
Identify the monomer of nylon – 2.
(a) adipic + Hexamethylene tetramine
(b) caprolactam
(c) vinyl chloride
(d) chioroprene
Answer:
(b) caprolactam
Question 117.
Which of the following is a fibre?
(a) nylon
(b) neoprene
(c) PVC
(d) bakelite
Answer:
(a) nylon
Question 118.
Identify the food preservative which is most commonly used by food producers?
(a) sodium cloride
(b) sodium sulphate
(c) baking soda
(d) benzoic acid
Answer:
(a) sodium cloride
![]()
Question 119.
Which of the following act as an antiseptic and disinfectant respectiely?
(a) 0.2% phenol, 1% phenol
(b) 1% phenol, 0.2% phenol
(c) 2% phenol, 20% phenol
(d) 20% phenol, 2% phenol
Answer:
(a) 0.2% phenol, 1% phenol
Question 120.
Identify the narcotic which is used as an analgesic.
(a) phenol
(b) equanil
(c) morphine
(d) cetrizine
Answer:
(c) morphine
Question 121.
What type of drug pencillin is?
(a) anaesthetic
(b) antibiotic
(c) antipyretic
(d) analgesic
Answer:
(b) antibiotic
Question 122.
Ranitidine is used as an …………
(a) antioxidant
(b) antiseptic
(c) antacid
(d) antibiotic
Answer:
(c) antacid
Question 123.
Aspirin is chemically named as ………….
(a) methyl salicylate
(b) ethyl salicylate
(c) o – hydroxy benzoic acid
(d) acetyl salicylic acid
Answer:
(d) acetyl salicylic acid
![]()
Question 124.
Which of the following can be used an analgesic without causing addiction and any modification?
(a) morphine
(b) n – acetyl paraminophenol
(c) diazepam
(d) tetra hydro catenol
Answer:
(c) diazepam
Question 125.
Tranquilisers are substances used for the treatment of ……………..
(a) cancer
(b) AIDS
(c) mental diseases
(d) blood infection
Answer:
Question 126.
Which of the following represents a synthetic detergent?
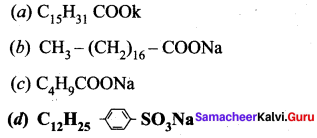
Answer:
![]()
Question 127.
Which of the following represents a soap?
(a) C17H35COOk
(b) C17H35COOH
(c) C15H31COOOH
(d) (C17H35COO)2Ca
Answer:
(a) C17H35COOk
Question 128.
Which of the following drug is an analgesic?
(a) iodex
(b) valium
(c) analgin
(d) quinine
Answer:
(c) analgin
Question 129.
An antipyretic is …………
(a) chioro quinine
(b) paracetamol
(c) morphine
(d) ranitidine
Answer:
(b) paracetamol
Question 130.
Streptomycin is effective in the treatment of ……………
(a) tuberculosis
(b) malaria
(c) typhoid
(d) cholera
Answer:
(a) tuberculosis
Question 131.
A drug effective in the treatment of pneumonia, bronchitis etc is ………………….
(a) streptomycin
(b) aspirin
(c) penicillin
(d) paracetamol
Answer:
(c) penicillin
![]()
Question 132.
The substances which affect the central nervous system and induce sleep are called ………………..
(a) tranquilizers
(b) analgesics
(c) antioxidants
(d) antipyretic
Answer:
(a) tranquilizers
Question 133.
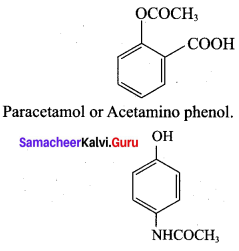
The correct structure of the drug paracetamol is ……………
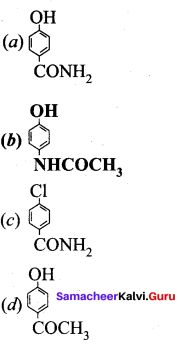
Answer:
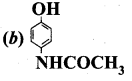
Quesiton 134.
Which of the following acts as an antioxidant in edible oils?
(a) Vitamin B
(b) Vitamin C
(c) Vitamin D
(d) Vitamin E
Answer:
(d) Vitamin E
Question 135.
Which of the following is an antidiabatic drug?
(a) insulin
(b) inulin
(c) chioroquine
(d) aspirin
Answer:
(a) insulin
Question 136.
Which of the following terms means pain killer?
(a) antibiotics
(b) analgesic
(c) antiseptic
(d) antioxidant
Answer:
(b) analgesic
![]()
Question 137.
The artificial sweetener containing chlorine that has the appearance and taste as the sugar and is stable at cooking temperature is …………….
(a) aspartame
(b) saccharin
(c) sucralose
(d) alitame
Answer:
(c) sucralose
Question 138.
The role of phosphate in detergent powder is …………..
(a) control pH level of the detergent water mixture
(b) remove Ca2+ and Mg2+ ions from water that causes hardness of water
(c) provide whiteness to the fabric
(d) more soluble in soft water
Answer:
(b) remove Ca2+ and Mg2+ ions from water that causes hardness of water
Question 139.
Which among the following is not an antibiotic?
(a) erythromycin
(b) oxytocin
(c) penicillin
(d) tetracycline
Answer:
(b) oxytocin
Question 140.
Commonly used antiseptic ‘dettoP is a mixture of …………..
(a) O – chloro phenozylenol + terpeneol
(b) O – cresol + terpenol
(c) phenol + terpeneol
(d) chioroxylenol + terpeneol
Answer:
(d) chioroxylenol + terpeneol
II. Fill in the blanks.
- The specific treatment of a disease using medicine is known as ……………..
- The drug which interacts with macro molecular targets such as proteins to produce a therapautic and useful biological response is called ………………
- ……………..is a substance that is used to modifS’ or explore physiological systems for the benefit of the recepient.
- Higher the value of …………….. safer is the drug
- The medicines that have ability to kill the pathogenic bacteria are grouped as ……………..
- Proteins which act as biological catalysts are called …………….. and those which are important for communication systems are called ……………..
- Many bacteria need …………….. in order to produce an important coenzyme, folic acid
- When adenosine binds to the adenosine receptors, it induces ……………..
- Morphine that used as a pain killer suppress the …………….. that causes pain.
- Histames stimulate the secretion of …………….. by activating the receptor in the stomach wall.
- …………….. acts on the central nervous system by blocking the neuro transmitter dopamine in the brain.
- …………….. reduce the pain without causing impairment of consciousness.
- …………….. are drugs that used to reduce fever and prevent platelet coagulation
- …………….. relieve pain and produces steeps and they are additive.
- …………….. neutralise the acid in the stomach that causes acidity.
- …………….. cause a controlled and reversible loss of consciousness by affecting central nervous SyStem.
- …………….. anaesthetics are often used for major surgical procedures.
- …………….. provide relief from allergic effects.
- …………….. inhibits bacterial cell wall biosynthesis.
- …………….. inhibits bacterial enzyme DNA gyrase.
- …………….. stop or slow down the growth of microorganisms applied to living tissues.
- …………….. stop or slow down the growth of microorganisms used on inanimate objects.
- The substances which are not naturally a part of the food and added to improve the quality of food are called ……………..
- Flavouring agents added to food enhance the …………….. of the food.
- …………….. are substances which retard the oxidative deteriorations of food.
- Synthetic compounds which imprint a sweet sensation and possess no or negligible nutritional value are called ……………..
- Chemically soap is a …………….. or …………….. salt of higher fatty acids.
- …………….. is a sodium salt alkyl hydrogen sulphate or alkyl benzene suiphonic acid.
- The quality of soap is described interrns of …………….. and the …………….. quantity in the soap better is its quality
- …………….. become soft on heating and hard on cooling and they can be remoulded.
- …………….. donot become soft on heating but set to an infusible mass upon heating.
- In the manufacture of Teflon. the monomer used is ……………..
- …………….. is used as a substitute of wool for making blankets, sweaters
- …………….. is a monomer which polymerises to give nylon – 6.
- Para hydroxyl methyl phenols poíymerises to give a linear polymer called ……………..
- The monomer of natural rubber is ……………..
- For the vulcanization of natural rubber …………….. is used and heated to 100° 150°C.
- …………….. polymers are used in medical field such as surgical sutures, plasma substitute.
- A drug that binds to the receptor site should inhibit its natural function is called ……………..
- …………….. reduces fever by causing the hypothalamus to override a prostaglandin-induced increase in temperature.
Answer:
- chemotheropy
- medicine
- Drug
- therapeutic index
- antibiotics
- enzymes, receptors
- PABA
- sleepiness
- neuro transmitters
- HCI
- Tranquilizers
- Analgesics
- Antipyretic
- Narcotic Analgegics (or) opioids
- Antacids
- General anaesthetics
- Inhalational general
- Antihistamines
- Antimicrobials
- Fluoroquinolones
- Antiseptic
- Disinfectants
- food additives
- aroma
- Antioxidant
- artificial sweatness
- sodium, potassium
- Detergent
- TFM, TFM
- Thermoplastic
- Thermosetting
- tetra fluoroethylene
- orIon (or) PAN
- Caprolactam
- novolac
- cis – isoprene (OR) 2 – methyl buta- 1, 3 – diene
- sulphur
- Biodegradable
- antagonists
- Non steroidal anti inflammatory drugs (or) NSAIDS
III. Match the following
Question 1.
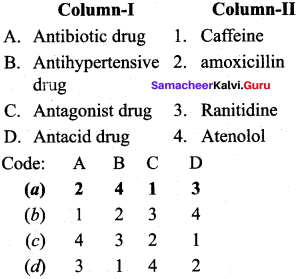
Answer:
(a) 2 4 1 3
Question 2.
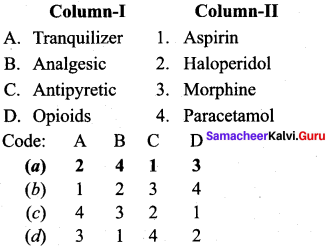
Answer:
(a) 2 4 1 3
Question 3.
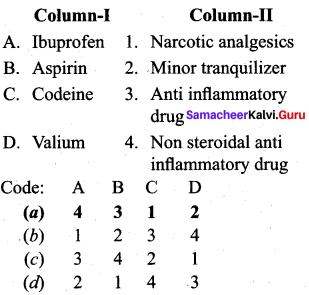
Answer:
(a) 4 3 1 2
Question 4.
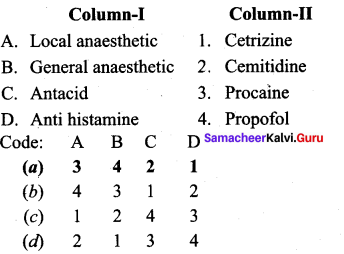
Answer:
(a) 3 4 2 1
Question 5.
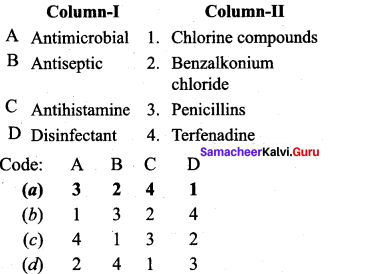
Answer:
(a) 3 2 4 1
Question 6.
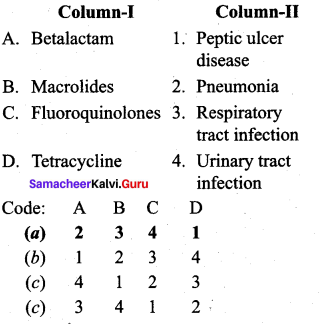
Answer:
(a) 2 3 4 1
Question 7.
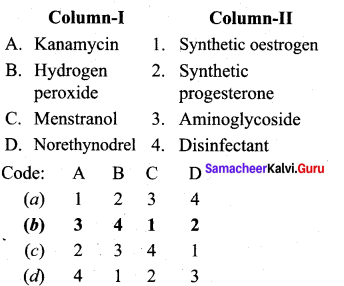
Answer:
(b) 3 4 1 2
Question 8.
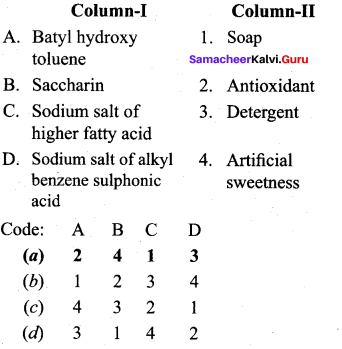
Answer:
(a) 2 4 1 3
Question 9.
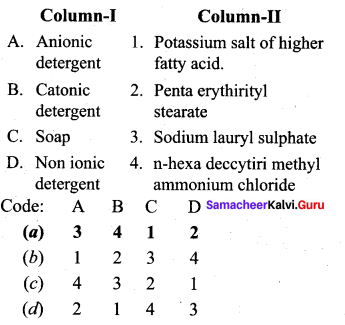
Answer:
(a) 3 4 1 2
Question 10.
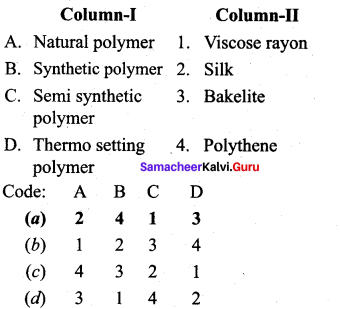
Answer:
(a) 2 4 1 3
Question 11.
Answer:
(a) 2 4 1 3
Question 12.
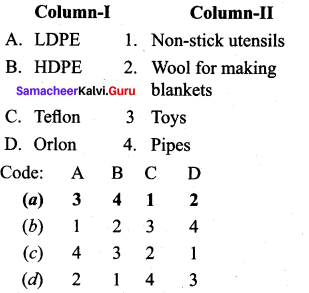
Answer:
(a) 3 4 1 2
Question 13.
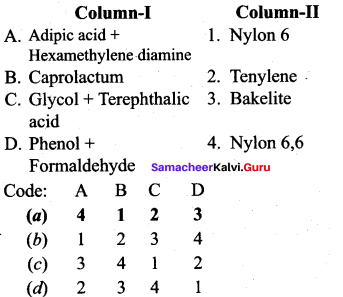
Answer:
(a) 4 1 2 3
Question 14.
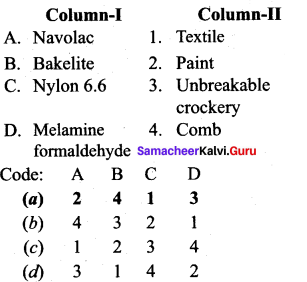
Answer:
(a) 2 4 1 3
Question 15.
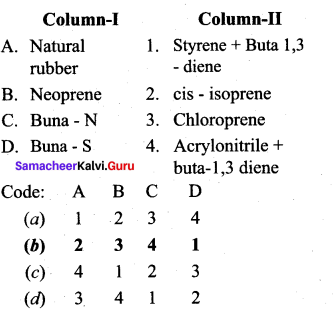
Answer:
(b) 2 3 4 1
Question 16.
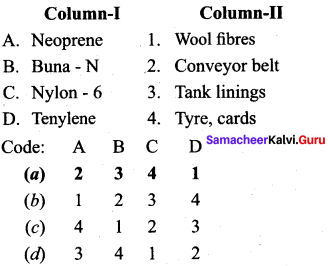
Answer:
(a) 2 3 4 1
IV. Assertion and reasons.
Question 1.
Assertion(A): Higher the value of therapeutic index, safer the drug.
Reason (R): Therapeutic index is defined as the ratio between the maximum tolerated dose of a drug and the minimum curative dose.
(a) Both A and R are correct and R Is the correct explanation of A.
(b) Both A and R are correct but R dOes not explains A.
(c) A is correct but R is wrong.
(d) A is.wrong but R is correct.
Answer:
(a) Both A and R are correct and R Is the correct explanation of A.
Question 2.
Assertion(A): In all living systems, the biochemical reactions are catalysed by enzymes. This principle is applied to kill many pathogens.
Reason (R): The enzyme actions are highly essential for normal functioning of the system.
(a) Both A and R are correct and R explains A.
(b) Both A and R are correct but R does not explain A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R explains A.
Question 3.
Assertion(A): The drugs acts as an inhibitor to the enzyme catalyst.
Reason (R): A drug molecule that has a similar geometry (shape) as the substrate is administered, it can also bind to the enzyme and inhibit its activity.
(a) Both A and R are correct and R explains A.
(b) Both A and R are correct but R does not explain A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R explains A.
![]()
Question 4.
Assertion(A): Aspirin is an antipyretic and useful in the prevention of heart attacks.
Reason (R): Aspirin reduces fever and also prevent platelet coagulation.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are correct but R does not explain A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R is the correct explanation of A.
Question 5.
Assertion(A): Opioids produces coma and even death.
Reason (R): Opioids releive pain and produce sleep and drugs are addictive and also poisonous in nature.
(a) Both A and R are correct and R explains A.
(b) Both A and R are correct but R does not explain A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R explains A.
Question 6.
Assertion(A): Milk of magnesia and aluminium hydroxide are usually used as antacids.
Reason (R): Mg(OH)2 and Al(OH)3 are weak bases and they neutralise the acid in the stomach that causes acidity.
(a) Both A and R are correct and R explains A.
(b) Both A and R are correct but R does not explain A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R explains A.
![]()
Question 7.
Assertion(A): Procaine and Lidocaine are local anaesthetics and cause loss of sensation in the area in which it is applied without losing consciousness.
Reason (R): They block pain perception that is transmitted via peripheral nerve fibres to the brain.
(a) Both A and R are correct and R explains A.
(b) Both A and R are correct but R does not explain A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R explains A.
Question 8.
Assertion(A): Antioxidant such as butyl hydroxy toluene (BHT) and butylated hydroxy anisole (BHA) are added as good additives.
Reason (R): Antioxidants retard the oxidative deterioration of food which contain fat and oils is easily oxidised and turn rancid.
(a) Both A and R are correct and R is the correct explanation of A.
(b) Both A and R are correct but R does not explain A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R is the correct explanation of A.
Question 9.
Assrtion(A): Saccharin, sucralose are artificial sweeteners.
Reason (R): Synthetic compounds which imprint a sweet sensation and possess no or negligible nutritional value are called artificial sweeteners.
(a) Both A and R are correct and R does not explains A.
(b) Both A and R are correct and R explains A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(b) Both A and R are correct and R explains A.
Question 10.
Assertlon(A): Sulphur dioxide and suiphites are also used as food additive.
Reason (R): They act as antimicrobial agents, antioxidant and enzyme inhibitors.
(a) Both A and R are correct but R does not explains A.
(b) Both A and R are correct and R explains A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(b) Both A and R are correct and R explains A.
Question 11.
Asscrtion(A): During soap preparation, common salt is added to the reaction mixture.
Reason (R): Common salt decreases the solubility of soap and it helps to precipitate out from the aqueous solution.
(a) Both A and R are correct and R explains A.
(b) Both A and R are correct but R does not explain A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R explains A.
![]()
Question 12.
Assertion(A): Higher the TFM quantity in the soap, better is its quality.
Reason (R): The quality of the soap is described in terms of total fatty matter (TFM value). Grade I soap should have 76% minimum TFM value.
(a) Both A and R are correct and R explains A.
(b) Both A and R are correct but R does not explain A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R explains A.
Question 13.
Assertion(A): Natural rubber becomes strong and elastic when heated with sulphur.
Reason (R): Natural rubber is mixed with 3 – 5% sulphur and heated at 100 – 150°C causes cross linking of the cis – 1, 4 – polyisoprene chains through disulphide – s – s bonds.
(a) Both A and R are correct and R explains A.
(b) Both A and R are correct but R does not explain A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R explains A.
Question 14.
Assertion(A): Artificial sweeteners are added to the food to control the intake of calories.
Reason (R): Most of the artificial sweeteners are inert and do not metabolise in the body,
(a) Both A and R are correct and R explains A.
(b) Both A and R are correct but R does not explain
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R explains A.
Question 15.
Assertion(A): Penicillin (G) is an antihistamine.
Reason (R): Penicillin G is effective against gram positive as well as gram negative bacteria.
(a) Both A and R are correct and R explains A.
(b) Both A and R are correct but R does not explain A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(d) A is wrong but R is correct.
Question 16.
Assertlon(A): Enzymes have active sites that hold substrate molecule for a chemical reaction.
Reason (R): Drugs compete with natural substate by attaching covalently to the active site of enzyme.
(a) Both A and R are correct and R explains A.
(b) Both A and R are correct but R does not explain A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(c) A is correct but R is wrong.
Question 17.
Assertion(A): Transparent soaps are made by dissolving soaps in ethanol.
Reason (R): Ethanol made things invisible.
(a) Both A and R are correct and R explains A.
(b) Both A and R are correct but R does not explain A.
(c) A is correct but R Is wrong.
(d) A is wrong but R is correct.
Answer:
(c) A is correct but R Is wrong.
![]()
Question 18.
Assertion(A): Sodium chloride is added to precipitate soap after saponification.
Reason (R): Hydrolysis of esters of long chain fatty acids by alkali produces soap in colloidal form.
(a) Both A and R are correct and R explains A.
(b) Both A and R are correct but R does not explain A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R explains A.
Question 19.
Assertlon(A): Aspirin has antipyretic properties.
Reason (R): Aspirin gives relief from pain.
(a) Both A and R are correct and R explains A.
(b) Both A and R are correct but R does not explain A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R explains A.
Question 20.
Assertlon(A): Bithional is added to soap as an antiseptic.
Reason (R): Bithional is a suipha drug and destroy bacteria.
(a) Both A and R are correct and R explains A.
(b) Both A and R are correct but R does not explain A.
(c) A is correct but R is wrong.
(d) A is wrong but R is correct.
Answer:
(a) Both A and R are correct and R explains A.
VI. Find out the correct pair.
Question 1.
Ampicillin, amoxicillin, methiceillin, cetrizine, cephalosporin.
Answer:
Cetrizine. It is an antihistamine whereas others belongs to penicillin group.
Question 2.
Aluminium hydroxide, magnesium hydroxide, erythromycin, cimetidine, ranitidine.
Answer:
Erythromycin. It is antimicrobial whereas others are antacids.
![]()
Question 3.
Halo peridol, clozapine, aiprazolam, aspirin, diazepam.
Answer:
Aspirin. It is an analgesic and antipyretic whereas others are anaesthetics.
Question 4.
Acetamino phenol, ibuprofen, aspirin, morphine.
Answer:
Morphine. It is an opioids (narcotic analgesic) whereas others are non narcotic analgesics.
Question 5.
Morphine, heroin, hydrocodone, codeine, ibuprofen.
Answer:
Ibuprofen. It is a non narcotic analgesic whereas others are narcotic analgesics.
Question 6.
Procaine, lidocaine, cemitidine, propofol, iso flurane.
Answer:
Ccmitidine. It is an antacid whereas others are anaesthetics.
Question 7.
Omeprazole, rabeprazole, iso flurane, ranitidine, cemitidine.
Answer:
Isoflurane. It is an anaesthetic whereas others are antacids.
Question 8.
Cetrizine, levocetrizine, trefenadine, ampicillin, desloratatide.
Answer:
Ampicillin. It is antimicrobial whereas others are antihistamines.
Quesiton 9.
Penicillin, ampicillin, cephalosorins, hydrogen peroxide, carbapenems.
Answer:
Hydrogen peroxide. it is an antiseptic where as other are antimicrobials.
Question 10.
Hydrogen peroxide, povidone – iodine, chlorine compounds, benzalkonium chloride.
Answer:
Chlorine compounds. It is a disinfectant whereas others arc antiseptic.
Question 11.
Ethynylestradiol, menstranol, hydrogen peroxide, norethindrone, norethynodrel.
Answer:
Hydrogen peroxide. It is an antiseptic and a disinfectant whereas otheres are antifertility drugs.
![]()
Question 12.
Sodium benzoate, salt of sorbic acid, acetic acid, sodium bi carbonate, sodium meta suiphite.
Answer:
Sodium bi carbonate. It is a baking soda whereas others are food preservatives.
Question 13.
BIIT, BHA, SO2, Vitamin E, sorbitol.
Answer:
Sorbitol. It is a sugar substituent where as others are antioxidants.
Question 14.
Saccharin, butyl hydroxy toluene, aspartane, sucralose, alitaine.
Answer:
Butyl hydroxy toluene, It is an antioxidant where as others are artificial sweetening agents.
Question 15.
Cellulose, polyester, silk.
Answer:
Polyester. It is a synthetic polymer whereas others are natural polymer.
Question 16.
PVC, polythene, LDPE, cellulose, HDPE, bakelite
Answer:
Cellulose, It is a natural polymer whereas others are synthetic polymers.
Question 17.
Polythcne, PVC, Bakelite, polystrene.
Answer:
Bakelite. It is thermosetting plastic whereas others are thermoplastic.
Question 18.
Nylon 66, polyethylene, PVC, teflon.
Answer:
Nylon 66. It is a condensation polymer whereas others are addition polymers.
Question 19.
Neoprene, bakelite, Buna – S, Buna – N.
Answer:
Bakelite. It is a thermosetting plastic whereas others are synthetic rubber.
Question 20.
Nylon 66, Nylon 6, terylene, teflon, bakelite, melamine.
Answer:
Teflon. It is an additional polymer whereas others are condensation polymers.
Samacheer Kalvi 12th Chemistry Chemistry in Everyday Life 2 mark Questions and Answers
Question 1.
Define the term
- medicine
- chemotherapy
Answer:
1. Medicine:
The drug which interacts with macromolecular targets such as proteins to produce a therapeutic and useful biological response is called medicine.
2. Chemotherapy:
The specific treatment of a disease using medicine is known as chemotherapy.
Question 2.
Define the term therapeutic index.
Answer:
1. Therapeutic index is defined as the ratio between the maximum tolerated dose of a drug (above which it becomes toxîc) and the minimum curative dose (below which the drug is ineffective).
2. Higher the value of therapeutic index, safer is the drug.
Question 3.
Write about the classification of drugs based on the target system.
Answer:
1. In this classification, the drugs are grouped based on the biological system that they target in the recepient. For example, the antibiotics streptomycin and erthyromycin inhibit the protein synthesis in bacteria and are classified in the same group.
2. However their mode of action is different. Streptomycin inhibits the initiation of protein synthesis, while erythromycin prevents the incorporation of new amino acids to the protein.
Question 4.
Explain about the classification of drug based on the site of action.
1. The drug molecule interacts with biomolecules such as enzymes, receptors which are referred as drug targets. The drug is classified based on the drug target with which it binds.
2. This classification is highly specilic compared to others. These compounds often have a common mechanism of action, as the target is the same.
![]()
Question 5.
What are
- antagonists
- agonists.
Answer:
- The drugs which binds to the receptor site and inhibit its natural function are called antagonists.
- There are drugs which mimic the natural messenger by switching on the receptor. Those type of drugs are called agonists.
Question 6.
What is the difference between an agonist and antagonist?
Answer:
- Agonist and antagonist act in opposite directions. Agonist is a substance which combines with cell receptor to produce some reaction that is typical for that substance.
- On the other hand antagonist is the chemical which opposes or reduces the natural function.
Question 7.
Explain the action of agonist and antagonist with proper example.
Answer:
When adenosine binds to the adenosine receptors, it induces sleepiness. So adenosine is an agonist. On the other hand, the antogonist drug coffeine binds to the adenosine receptor and makes it inactive. This results in the reduced sleepiness (wakefulness).
Question 8.
Why ranitine is a better antacid than magnesium hydroxide?
Answer:
To treat acidity, weak base such as magnesium hydroxide is used. But this weak base make the stomach alkaline and trigger the production of much acid. This treatment only relieves the symptoms and does not control the cause. But ranitine stimulate the secretion of HCI by activating the receptor in the stomach wall which binds the receptor and inactivate them. So ranitine is a better antacid than magnesium hydroxide.
Question 9.
What is meant by non-steroidal anti inflammatory drugs? Give example.
Answer:
Non – steroidal anti inflammatory drugs reduces fever by causing the hypothalamus to override a prostaglandin – induced increase in temperature. eg., ibuprofen.
Question 10.
What are narcotic analgesics? Give examples.
Answer:
- Narcotic analgesics are opioids that relieve pain and produce sleep. These drugs are addictive. In poisonous dose, these produces coma and ultimately death. eg, morphine, codeine,
- These drugs are used for short term or long term relief of severe pain. Mainly used for post operative pain. Pain of terminal cancer.
![]()
Question 11.
What are general anaesthetics? Give example.
Answer:
- General anaesthetics are drugs cause a controlled and reversible loss of consciousness by affecting central nervous system. e.g., propofol, iso flurane.
- They are often used for major surgical procedures.
Question 12.
What are local anaesthetics? Give example. Mention its uses.
Answer:
- Local anaesthetics cause loss of sensation in the area in which it is applied without losing consciousness. They block pain perception that is transmitted via peripheral nerve fibre to the brain. e.g., procaine, lidocaine
- They are often used during minor surgical procedures.
Question 13.
Draw the structure of propofol? Mention its use.
Answer:
Propofol structure:
Question 14.
What are antihistamines? Give example and mention its use.
Answer:
- Antihistamines block histamine release from histamine – 1 receptors.
- eg., cetirizine, terfenadine, levocetirizine.
- It is used to provide relief from the allergic effects.
![]()
Question 15.
What are antimicrobials? Mention its function and its uses.
Answer:
- Antimicrobials inhibits bacterial cell wall biosynthesis.
- e.g., penicillin, ampicillin.
- It is used to treat skin infections, dental infections, ear infections, respiratory tract infections. Pneumonia, urinary tract infections and gonorrhoea.
Question 16.
Draw the structure of penicillin? Give its use.
Answer:
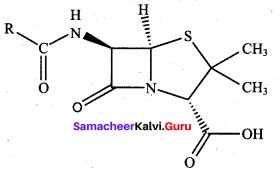
Penicillin is used to treat all type of infections pneumonia, urinary tract infections.
Question 17.
Draw the structure of ampicillin
Answer:
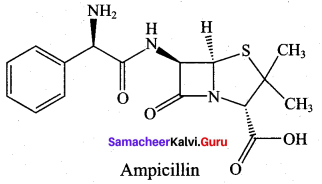
Question 18.
Write a note about macrolids.
Answer:
- Macrolids targets bacterial ribosomes and prevent protein production. e.g., erythromycin, azithromycin
- It is used to treat respiratory tract infections, genital, gastro intestinal tract and skin infections.
Question 19.
What are fluoroquinolones? Give its function and uses.
Answer:
- Fluoro quinolones inhibits bacterial enzyme DNA gyrase.
- e.g., clinafloxacin, ciprofloxacin
- It is used to treat urinary tract infections, skin infections and respiratory infections, pulmonary infections in cystic fibrosis.
![]()
Question 20.
What are tetracydines? Mention its function and uses.
Answer:
- Tetracyclines inhibit the bacterial protein synthesis via interaction with the 30 S subunit of the bacterial ribosome. eg., doxycycline, minocycline.
- It is used in the treatment of peptic ulcer disease, infections of the respiratory tract, cholera.
Question 21.
What are aminoglycosides? Give its function and uses.
Answer:
- Aminoglycosides bind to the 30 S subunit of the bacterial ribosome, thus stopping bacteria from making proteins.
- It is used to treat infections caused by gram negative bacteria.
Question 22.
What are food additives? Give example.
Answer:
- The substances which are not naturally a part of the food and added to improve the quality of food are called food additives.
- e.g., Aroma compounds, antioxidants, preservatives, stabilizers, food colours, buffering substances are food additives.
Question 23.
Explain about antioxidants.
Answer:
- Antioxidants are substances which retard the oxidative deteriotations of food. Food containing fats and oils is easily oxidised and turn rancid, .
- To prevent the oxidation of fats and oils, chemical BHT (butyl hydroxy toluene), BHA (butylated hydroxy anisole) are added as antioxidants.
- These materials readily undergo oxidation by reacting with free radicals generated by the oxidation of oils there by stop the chain reaction of oxidation of food.
- Sulphur dioxide, suiphites are also used as antioxidant and also act as antimicrobial agents and enzyme inhibitors.
Question 24.
What are sugarsubstituents? Give example.
Answer:
The compounds that are used like sugars for sweetening, but are metabolised without the influence of insulin are called sugar substituents. e.g., sorbitol, xylitol, mannitol.
![]()
Question 25.
What are artificial sweetening agents? Give example.
Answer:
Synthetic compounds which imprint a sweet sensation and possess no or negligible nutritional value are called artificial sweeteners. e.g., saccharin, aspartame, sucralose, alitame.
Question 26.
Define TFM value.
Answer:
- The quality of a soap is described in terms of total fatty matter (TFM value). It is defined as the total amount of fatty matter that can be separated from a sample after spliting with mineral acids.
- Higher the TFM value in the soap, better is its quality.
- As per BIS standards, Grade I soaps should have 76% minimum TFM value.
Quesiton 27.
Write a note about natural rubber and give Its structure.
Answer:
1. Rubber is a naturally occuring polymer. It is obtained from the latex that excludes from cuts in the bark of rubber tree.
2. The monomer unit of natural rubber is cis – iso prene (2 – methyl buta – 1,3 – diene). Thousands of isoprene units are linearly linked together in natural rubber. NaturaL rubber is not so strong (or) elastic. The properties of natural rubber can be modified by the process called vulcanization.
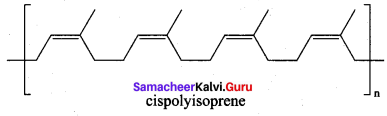
Question 28.
How is neoprene prepared? Give its use.
Answer:
1. The free radical polymerisation of the monomer 2 – chloro buta 1,3 – diene (chioroprene) gives neoprene.
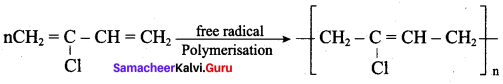
2. It is used in the manufacture of chemical container, conveyer belts.
Question 29.
How Is Buna – N prepared? Give its use.
Answer:
1. Buna – N is prepared by the polymerisation of acrylonitile and buta – 1, 3 – diene

2. It is used in the manufacture of hoses and tank linings.
![]()
Question 30
How would you prepare Buna – S? Give its use.
Answer:
Buna – S is prepared by the polymerisation of buta – 1, 3 – diene and styrene in the ratio of 3 : 1 in the presence of sodium.
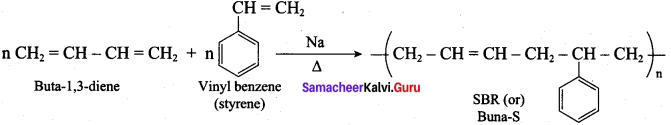
Uses:
It is used in making pneumatic tires in shoe heels and soles, and in gaskets.
Question 31.
How will you prepare PHBV? Give its use?
Answer:
1. The biodegrable polymer PHBV (Poly hydroxy butyrate-co hydroxyl valerate) is prepared by the polymerisation of monomers 3 – hydroxy butanoic acid and 3 – hydroxy pentanoic acid.
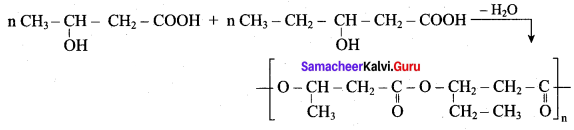
2. It is used in orthopacdic devices añd in controlled release of drugs.
Question 32.
How would you prepare Nylon – 2 – Nylon – 6 polymers?
Answer:
Nylon – 2 – Nylon 6 is a co polymer which contains polyamide linkages. It is obtained by the condensation polymerisation of monomers glycine and E-amino caproic acid.

Question 33.
What are natural and synthetic polimers? Give two examples of each type.
Answer:
Natural polymers:
Polymers which are found in nature, i.e., in animal and plants are called natural polymers. For example, proteins, starch, cellulose etc.
Synthetic polymers:
Man – made polymers are called synthetic polymers. For example plastics, synthetic fibres.
Question 34.
Distinguish between the terms homopolymer and copolymer and give an example of each.
Answer:
Homopolyers:
Polymers whose repeating structural units are dervied from only one type of monomer units are called homopolymers. For example, polythene, PVC, PAN etc.
Copolymers:
Polymers whose repeating units are derived from two or more types of monomer molecules are called co-polymers. For example, l3una – S, Buna – N, Nylon 6, 6 etc.
![]()
Question 35.
How can you differentiate between addition and condensation polymerisation?
Answer:
Addition polymerisation:
In this type of polymerisation, a large number of molecules of same or different monomers simply add to the other unit, leading to the formation of macromolecule. Addition polymerisation generally occurs among molecules containing double and triple bonds.
Condensation polymerisation:
In this type of polymerisation two or more bifunctional molecules undergo a series of independent condensation reactions usually with the elimination of simple molecules like water, alcohol, ammonia etc.
Question 36.
What are the monomeric repeating units of Nylon – 6 and Nylon 6, 6?
Answer:
Nylon 6 – Caprolactam. Nylon 6,6 – Adipic acid and Hexamethylenediamine
Question 37.
Write the names and structure of the monomers of the following polymers:
- Buna – S
- Buna – N
- Dacron
- Neoporene
Answer:
1. Butadiene, CH2 = CH – CH = CH2 ; Styrene, C6H5 – CH = CH2
2. Butadiene, CH2 = CH – CH = CH2 ; Acrylonitrile, 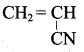
3. Terephthalic acid, ; Ethylene glycol (Ethane – 1, 2 – diol)
; Ethylene glycol (Ethane – 1, 2 – diol)
4. Chloroprene,![]() ; 2 – Chloro – 1, 3 – butadine is the monomer of neoprene.
; 2 – Chloro – 1, 3 – butadine is the monomer of neoprene.
Question 38.
How is dacron obtained from ethylene glycol and terephthalic acid?
Answer:
Dacron is obtained by condensation polymerisation of ethylene glycol and terephthalic acid with the elimination of water molecules:
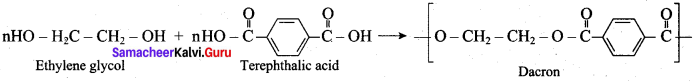
Question 39.
What is a biodegradable polymer? Give an example of a biodegradable aliphatic polyester.
Answer:
Polymers which disintegrate by themselves over a period of time due to environmental degradation by bacteria, etc. are called biodegradable polymers. Example: PHBV (poly hydroxy butrate – co – β hydroxyvalcrate)
Question 40.
What is the difference between elastomers and fibres? Give one example of each.
Answer:
Elastomers
- These are rubber like solids with elastic properties.
- These are held by the weak inter – molecular forces.
- Example: Buna-S and Buna-N.
Fibres
- These are th thread forming solids which possess high tensile strength and high modulus.
- These are held together by strong intermolecular forces like hydrogen bonding.
- Example: Nylon 6, 6 and polyesters (terylene)
Question 41.
What are thermoplastics and thermosetting polymers? Give one example of each.
Answer:
Thermoplastics:
Thermoplastics are linear polymers which can be repeatedly softened on heating and hardened on cooling and hence can be used again and again without any change in chemical composition and mechanical strength.
Thermosetting polymers:
Thermosetting polymers, are permanently setting polymers. On heating in a mould, they get hardened and set and cannot be softened again. This hardening on heating is due to cross linking between different polymeric chains which give rise to a three dimensional network solid. Example – Bakelite.
![]()
Question 42.
Differentiate between addition and condensation polymers based on the mode of polyrnerisation. Give one example of each type. 1
Answer:
Addition polymers
- They are formed by adding monomers to a growing polymer chaìn without loss of any molecule.
- They are formed from unsaturated compounds.
- Example: Polyethene, polypropene.
Condensation polymers
- They are formed by combining monomers together with the loss of small molecules like H2O, NH3, CO2 etc.
- Monomers have di or polyfunctional groups.
- Example: Nylon – 6, 6, Nylon – 6, Terylene.
Question 43.
Distinguish between ‘chain growth potymerisatlon and step growth polymerisation’ and give one example of each.
Answer:
Chain growth polymerisation
- Only one repeating unit is added at a time.
- Reaction is fast and polymer is formed at once. Example – polythene.
Step growth polymerisatlon
- Any two species present can react.
- Polymer is formed in gradual steps. Examply – Nylon-6, 6.
Question 44.
How are biopolymers more beneficial than synthetic polymers?
Answer:
Durability of synthetic polymers is advantageous, however it presents a serious waste disposable problem. In renewal of the disposable problem, biodegradable polymers are useful to us. Biopolymers arc safe in use. They disintegrate by themselves in biological system during a certain period of time by enzymatic hydrolysis and to some extent by oxidation and hence, are biodegradable. As a result, they do not cause any pollution.
Question 45.
Give the method of preparation of polyacrylonitrile?
Answer:
The addition polymerisation of acrylonitrile in the presence of a peroxide catalyst leads to the formation of polycrylonitrile. It is used as a substitute for wool in making fibres such as orlon or acrilan.

Samacheer Kalvi 12th Chemistry Chemistry in Everyday Life 3 mark Questions and Answers
Question 1.
Draw the structures of
- Suiphanilamide
- p – nltro benzoic acid
Answer:
1. Suiphanilamide
2. p – nitro benzoic acid:
Question 2.
Draw the structure of
- Adenosine (Agonist)
- Caffeine (Antagonist)
Answer:
1. Adenosinc (Agonist)
2. Caffeine (Antagonist)
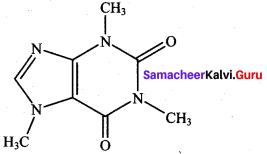
Question 3.
Explain about
- Analgesics
- Antlinflammatory drugs
- Antipyretlcs
Answer:
1. Analgesics:
They alleviate pain by reducing local inflammatory response. They reduce the pain without causing impairment of consciousness.
Example: Paracetamol (Crocin).
2. Anti inflammatory drug:
They are used for short term pain relief and for modest pain like head ache, muscle strain, bruising or arthritis.
Example: Ibuprofen, Aspirin.
3. Antipyretics:
These drugs have many effects such as reducing fever, and preventing platelet coagulation.
Example: Aspirin.
![]()
Question 4.
Explain about anaesthetics with their types.
Answer:
1. Local anaesthetics: It causes loss of sensation in the area in which it is applied without losing consciousness. They block pain perception that is transmitted via peripheral nerve fibres to the brain. Example: Procaine, Li do Caine. They are often used during minor surgical procedures.
2. General anaesthetics:
They cause a controlled and reversible loss of consciousness by affecting central nervous system.
Example: Propofol, Isoflurane. They are often used for major surgical procedures.
Question 5.
Draw the structure of
- procaine
- Lidocaine
Answer:
1. Procaine
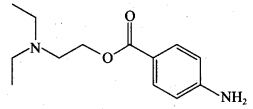
2. Lidocaine
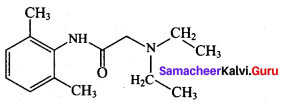
Question 6.
Explain about antacids?
Answer:
- Antacids neutralise the acid in the stomach that causes acidity.
- They are used to relieve burning sensation in the chest, throat area caused by acid reflux. Example – Milk of magnesia, alumminium hydroxide, Ranitidine, Cemitidino, Omeprazole, Rabeprazole.
Question 7.
Distinguish between Antiseptic and Disinfectants.
Answer:
1. Antiseptic:
They are the drugs used to stop (or) slow down the growth of micro organism and they are applied to living tissue (body). Example: H2O2.
2. Disinfectant:
They are the drugs used to stop or slow the growth of micro organism and they are applied on inanimate objects (non living surfaces). Example: Chlorine compounds.
Question 8.
What are the advantages of food additives?
Answer:
- Uses of preservatives reduce the product spoilage and extend the shelf-life of food.
- Addition of vitamins and minerals reduces the mall nutrient.
- Flavouring agents enhance the aroma of the food.
- Antioxidants prevent the formation of potentially toxic oxidation products of lipids and other food constituents.
![]()
Question 9.
Differentiate soap and detergents?
Answer:
Soap
- Soaps are sodium or potassium salt of long chain fatty acid.
- Soaps are made from animal (or) plant fats and oils.
- Soaps have lesser cleansing action.
- Soaps are bio degradable.
- Soaps are less effective in hard water.
- They have a tendency to form a scum in hard water.
- Example: Sodium palmitate.
Detergent
- Detergent is sodium salt of alkyl hydrogen sulphate or alkyl benzene suiphonic acid.
- Detergents are made from petrochemicals.
- Detergents have more cleansing action.
- Detergents are non – bio degradable.
- Detergents are more effective even in hard water.
- They do not form scum with hard water.
- Example: Sodium lauryl sulphate.
Question 10.
What is LDPE? Give its preparation and uses.
Answer:
1. LDPE is low density poiy ethylene. It is formed by heating ethene at 2000 to 300°C under oxygen as a catalyst. This reaction follows free radical mechanism. The peroxide formed from oxygen acts as a free radical initiator.

2. LDPE is used as insulation for cables, making toys.
Question 11.
What is HDPE? Cive its preparation and use.
Answer:
- IIDPE is high density polyethylene. it is prepared by the polymerisation of ethylene at. 373k and 6 to 7 atm. using zeiglar Natta Catalyst)
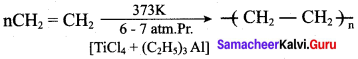
- It is used to make bottles, pipes.
Question 12.
What is Orlon? Give its preparation and use.
Answer:
1. Orlon is poiy acrylonitrite (PAN). It is prepared by the addition of polymerisation of vinyl cyanide using a peroxide initiator.
2.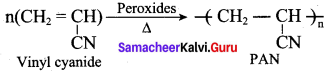
3. It is used as a substitute of wool for making blankets, sweaters etc.
Question 13.
How will you prepare Nylon 6,6.? Give its use.
Answer:
1. Nylon 6,6 can be prepared by mixing equimolar adipic acid and hexamethylene diaminc. With the elimination of water to form amide bonds.
2. 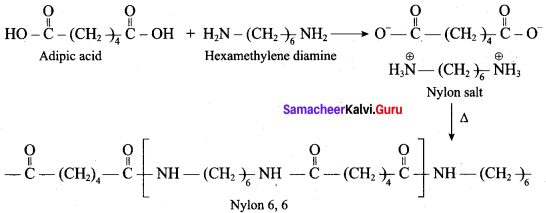
3. It is used in textiles, manufacture of cards.
Question 14.
How will you prepare Nylon – 6? Give its use.
1. Capro tactum on heating at 533k in an inert atmosphere with traces of water gives E amino caproic acid which polymerises to give Nylon 6.
2.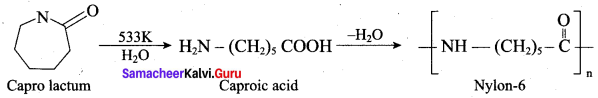
3. It is used in the manufacture of tyre cards, fabrics.
Question 15.
What is bakeite? How is it prepared? Give its uses.
Answer:
1. Bakelite is a thermo setting plastic. It is prepared from the monomers such as phenol and formaldehyde. The condensalion polymerisation take place in the presence of acid or base catalyst.
2. Phenol reacts with methanal to form ortho or para hydroxyl methyl phenols which on further reaction with phenol gives linear polymer called novolac. Novolac on further healing with formaldehyde undergoes cross linkages to form bakelite.
3.
4.
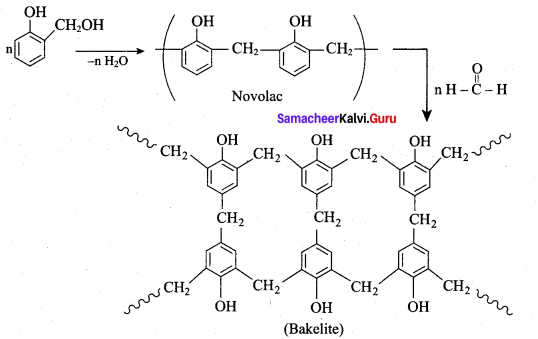
- Novolac is used in paints.
- Soft bakelites are used in making glue for binding laminated wooden planks and in varnishes.
- Hard bakelites are used to prepare combs, pens.
Question 16.
How Is melamlue prepared? Give its use?
Answer:
Melamine and formaldehyde are the monomers. They undergo condensation polymerisation to form melamine formaldehyde resin.
Uses : It is used in making unbreakable crockery.
Question 17.
How is urea formaldehyde prepared?
Answer:
It is formed by condensation polymerisation of the monomers urea and formaldehyde. Uses – it is used in decorative laminates, textiles, wrinkle resistant fabrics, paper and glue wood.
Question 18.
Mention one use of each of the following:
- Ranitidine
- Paracetamol
- Tincture of iodine.
Answer:
- Rnitidine is used as an antacid.
- Paracetamol is used to bring down the body temperature during high fever.
- Tincture of iodine is used as an antiseptic. It is 2-3% solution of iodine in alcohol and water.
Question 19.
Describe the following with suitable examples:
- Preservatives
- Artificial sweetening agents.
Answer:
1. Preservatives:
Preservatives are the substances which are used to prevent spoilage of food due to microbial growth. Examples – Sodium benzoate, Common salt.
2. Artificial sweetening agents:
These are the chemical substances which are used to create sweet taste in food items in place of sugar.
![]()
Question 20.
Give one important use of each of the following:
- Bithional
- Chioramphenicol
- Streptomycin
- Paracetamol
Answer:
- Bithional is added to soap so as to impart antiseptic properties to the soap.
- Chioramphenicol is a broad spectrum antibiotic used in curing typhoid, meningitis.
- Streptomycin is used for the treatment of T.B (Tuberculosis).
- Paracetamol is an antipyretic used in bringing down temperature in high fever.
Question 21.
What are detergents? How are they classified? Why are detergents preferred over soaps?
Answer:
Detergents are suiphonate or hydrogen sulphate salts of long chain hydrocarbons containing 12-18 carbon atoms.
Types of detergents
- Cationic detergents
- Anionic detergents
- Non-ionic detergents
Advantages of detergents over soaps: Unlike soaps they work well even with hard water. They can work well even in acidic water. They are more effective than soaps.
Question 22.
- What class of drug is Ranitidine?
- If water contains dissolved Ca2+ ions, out of soaps and synthetic detergents, which will you use for cleaning clothes?
- Which of the following is an antisepctic? 0.2% phenol, 1% phenol.
Answer:
- It is an antacid.
- In this case we use synthetic detergents because it give foam with hard water.
- 0.2% solution of phenol acts as antiseptic.
![]()
Question 23.
Define the following by giving one example of each:
- Antiseptics
- Antioxidants
- Narcotic analgesics
Answer:
- Antiseptics are the chemicals applied to the living tissues either to kill or prevent the growth of micro organisms. Example : dettol.
- Antioxidants are the compounds which retards the action of oxygen on food and reduces its rate of decomposition by oxidation. Example: BHA.
- Narcotic analgesics are the chemicals used for the relief of pst operative pain. Example – morphine.
Question 24.
In order to wash clothes which cleaning agent what will you prefer and why: soap or synthetic detergents? Give one advantage of soaps and synthetic detergents each.
Answer:
Soaps have straight hydrocarbon chains and are easily degraded by bacteria present in the sewage water and hence, do not cause water pollution. Most of the detergents are non – biodegradable and hence cause water pollution of rivers and waterways. So, one will prefer soap.
Question 25.
Name the action of the following on the human body.
- Aspirin
- Penicillin
- Phenacetin
- Morphine
- Analgin
- Luminal
- Seconal
- Streptomycin
Answer:
- Aspirin is an analgesic which is used for relieving pain. It also prevents heart attack.
- Penicillin is an antibiotic used against large number of infections caused by various cocci, gram positive bacteria, etc. It is an effective drug for pneumonia, bronchitis, sore throat.
- Phenacetin is an antipyretic drug used to bring down the temperature of body in high fever.
- Morphine is an strong analgesic. It is a narcotic drug. It cause addiction. It gives relief from acute pain, induce sleep and unconsciousness in higher doses.
- Analgin is an antipyretic and analgesic. It brings down the temperature of body in fever and give relief from pain.
- Luminal produces sleep and it is a habit forming drug. It is also called a sedative tranquilliser.
- Seconal is an antidepressant (tranquiliser). Sometimes the patients are highly depressed and loses self – confidence. This drug produces feeling of well being and improved efficiency.
- Streptomycin is used as an antibiotic. It is used to cure tuberculosis.
Samacheer Kalvi 12th Chemistry Chemistry in Everyday Life 5 mark Questions
Question 1.
Explain free radical polymerisation with example.
Answer:
1. When alkenes are heated with free radical initiator such as benzoyl peroxide, they undergo polymerisation reaction. For example, styrene polymerises to polystrene when it is heated with a peroxide initator. The mechanism involves the following steps.
2. Initiation – Formation of free radical.
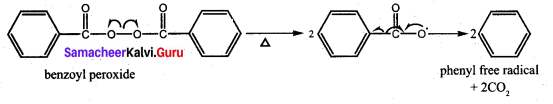
3. Propagation step.
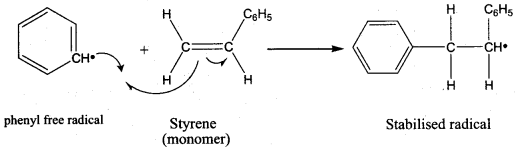
4. Chain growth will continue with the successive addition of several thousands of monomer units.
5. Termination:
The above chain reaction can be stopped by stopping the supply of monomer or by coupling of two chains or reaction with an impurity such as oxygen.
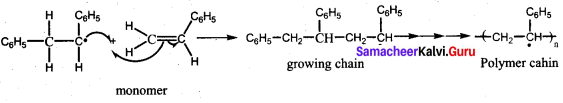
Question 2.
How are polymers classified on the basis of forces operating between their molecules? To which of these classes does nylon-6,6 belong?
Answer:
- Elastomers: The polymer chains are held together by weak intermolecular forces. Example – Buna – S, Buna – N, Neoprene.
- Fibres: They have strong forces of attraction. Example – Polymides, (Nylon 6,6), polyesters.
- Thermoplastics: They are long chain molecules capable of repeatedly softening on heating and hardening on cooling. Example – Polythene, polystyrene.
- Thermosetting plastics: They do not become soft on heating and cannot be remoulded. Example – Bakelite, Nylon – 6,6, belong to fibres.
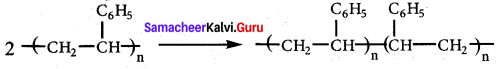
Common Errors
1. Medicines – Molecular fonnula are not given only structural fotmula are drawn.
Rectifications
1. It is written by counting the C, H, O, N in the compound. For example. Aspirin on Acetyt salicylic acid.