You can Download Samacheer Kalvi 10th Science Book Solutions Guide Pdf, Tamilnadu State Board help you to revise the complete Syllabus and score more marks in your examinations.
Tamilnadu Samacheer Kalvi 10th Science Solutions Chapter 7 Atoms and Molecules
Samacheer Kalvi 10th Science Atoms and Molecules Textual Solved Problems
I. Calculation of molar mass:
Question 1.
Calculate the gram molar mass of the following.
(i) H2O
(ii) CO2
(iii) Ca3(PO4)2
Solution:
(i) H2O
Atomic masses of H = 1, O = 16
Gram molar mass of H2O = (1 × 2) + (16 × 1) = 2 + 16
Gram molar mass of H2O = 18 g.
(ii) CO2
Atomic masses of C = 12, O = 16
Gram molar mass of CO2 = (12 × 1) + (16 × 2) = 12 + 32
Gram molar mass of CO2 = 44 g.
(iii) Ca3(PO4)2
Atomic masses of Ca = 40, P = 30, O = 16.
Gram molar mass of Ca3(PO4)2 = (40 × 3) + [30 + (16 × 4)] × 2
= 120 + (94 × 2)
= 120 + 188
Gram molar mass of Ca3(PO4)2 = 308 g.
You can Download Samacheer Kalvi 10th Science Guide Pdf Tamilnadu State Board help you to revise the complete Syllabus and score more marks in your examinations.
II. Calculation based on number of moles from mass and volume:
Question 1.
Calculate the number of moles in 46 g of sodium.
Solution:
Number of moles = \(\frac{\text { Mass of the element }}{\text { Atomic mass of the element }}\)
Atomic mass of the element = \(\frac { 46 }{ 23 }\) = 2 moles of sodium
Question 2.
5.6 litre of Oxygen at S.T.P?
Solution:
Given volume of O2 at,
Number of moles = \(\frac{\text { S.T.P. }}{\text { Molar volume at } \mathrm{S} . \mathrm{T} \cdot \mathrm{P}}\)
Molar volume at S.T.P = \(\frac { 46 }{ 23 }\) = 2 moles
Number of moles of oxygen = \(\frac{5.6}{22.4}\) = 0.25 mole of oxygen
Question 3.
Calculate the number of moles of a sample that contains 12.046 × 1023 atoms of iron?
Solution:
Number of moles = \(\frac{\text { Number of atoms of iron }}{\text { Avogadro’s number }}\)
= 12.046 × 1023 / 6.023 × 1023
= 2 moles of iron.
III. Calculation of mass from a mole.
Question 1.
0.3 mole of aluminium (Atomic mass of Al = 27).
Solution:
Number of moles = \(\frac{\text { Mass of Al }}{\text { Atomic mass of Al }}\)
Mass = No. of moles × atomic mass
So, mass of Al = 0.3 × 27 = 8.1 g.
Question 2.
2.24 litre of SO2 gas at S.T.P?
Solution:
Molecular mass of SO2 = 32 + (16 × 2) = 32 + 32 = 64
Number of moles of SO2 = \(\frac{\text { Given volume of } \mathrm{SO}_{2} \text { at } \mathrm{S} . \mathrm{T.P}}{\text { Molar volume } \mathrm{SO}_{2} \text { at } \mathrm{S} . \mathrm{T.P}}\)
= \(\frac{2.24}{22.4}=0.1 \mathrm{mole}\)
Number of moles = \(\frac{\text { Mass }}{\text { Molecular mass }}\)
Mass = No. of moles × molecular mass
Mass = 0.1 × 64
Mass of SO2 = 6.4 g.
Question 3.
1.51 × 1023 molecules of water
Solution:
Molecular mass of H2O = 18
Number of moles = \(\frac{\text { Number of molecules of water }}{\text { Avogadro’s number }}\)
= 1.51 × 1023 / 6.023 × 1023 = 1 / 4 = 0.25 mole
Number of moles = \(\frac{\text { Mass }}{\text { Molecular mass }}\)
0.25 = mass / 18
Mass = 0.25 × 18
Mass = 4.5 g.
Question 4.
5 × 1023 molecules of glucose?
Solution:
Molecular mass of glucose = 180
Mass of glucose = \(\frac{\text { Molecular mass } \times \text { number of particles }}{\text { Avogadro’s number }}\)
= (180 × 5 × 1023) / 6.023 × 1023
= 149.43 g.
IV. Calculation based on the number of atoms/molecules.
Question 1.
Calculate the number of molecules in 11.2 litre of CO2 at S.T.P
Solution:
Number of moles of CO2 = \(\frac{\text { Volume at S.T.P }}{\text { Molar volume }}\)
= \(\frac { 11.2 }{ 22.4 }\)
= 0.5 mole.
Number of molecules of CO2 = Number of moles of CO2 × Avogadro’s number
= 0.5 × 6.023 × 1023
= 3.011 × 1023 molecules of CO2.
Question 2.
Calculate the number of atoms present in 1 gram of gold (Atomic mass of Au = 198).
Solution:
Number of atoms of Au = \(\frac{\text { Mass of Au } \times \text { Avogadro’s number }}{\text { Atomic mass of Au }}\)
Atomic mass of Au = \(\frac{1}{198} \times 6.023 \times 10^{23}\)
Number of atoms of Au = 3.042 × 1021 g.
![]()
Question 3.
Calculate the number of molecules in 54 gm of H2O
Solution:
Number of molecules = \(\frac{(\text { Avogadro number } \times \text { Given mass })}{\text { Gram molecular mass }}\)
Number of molecules of water = 6.023 × 1023 × \(\frac { 54 }{ 18 }\)
= 18.069 × 1023 molecules.
Question 4.
Calculate the number of atoms of oxygen and carbon in 5 moles of CO2.
Solution:
- 1 mole of CO2 contains 2 moles of oxygen.
- 5 moles of CO2 contains 10 moles of oxygen
Number of atoms of oxygen = number of moles of oxygen × Avogadro’s number
= 10 × 6.023 × 1023 = 6.023 × 1024 atoms of Oxygen. - 1 mole of CO2 contains 1 mole of carbon
- 5 moles of CO2 contains 5 moles of carbon
No. of atoms of carbon = No.of moles of carbon × Avogadro’s number
= 5 × 6.023 × 1023 = 3.011 × 1024 atoms of Carbon.
V. Calculation based on molar volume
Calculate the volume occupied by:
Question 1.
2.5 mole of CO2 at S.T.P.
Solution:
\(\begin{array}{l}{\text { Number of moles of } \mathrm{CO}_{2}=\frac{\text { Given volume at S.T.P }}{\text { Molar volume at S.T.P }}} \\ {\qquad 2.5 \text { mole of } \mathrm{CO}_{2}=\frac{\text { Volume of } \mathrm{CO}_{2} \text { at } \mathrm{S} . \mathrm{TP}}{22.4}}\end{array}\)
Volume of CO2 at S.T.P = 22.4 × 2.5 = 56 litres.
Question 2.
3.011 × 1023 of ammonia gas molecules?
Solution:
Number of moles = \(\frac{\text { Number of molecules }}{\text { Avogadro’s number }}\)
= 3.011 × 1023 / 6.023 × 1023
= 2 moles
Volume occupied by NH3 = number of moles × molar volume
= 2 × 22.4 = 44.8 litres at S.T.P.
Question 3.
14 g nitrogen gas?
Solution:
Number of moles = \(\frac { 14 }{ 28 }\) = 0.5 mole
Volume occupied by N2 at S.T.P = No. of moles × molar volume
= 0.5 × 22.4
= 11.2 litres.
VI. Calculation based on % composition.
Question 1.
Calculate % of S in H2SO4
Solution:
Molar mass of H2SO4 = (1 × 2) + (32 × 1) + (16 × 4)
= 2 + 32 + 64
= 98 g.
\(\begin{array}{l}{\% \text { of } \mathrm{S} \text { in } \mathrm{H}_{2} \mathrm{SO}_{4}=\frac{\text { Mass of sulphur }}{\text { Molar mass of } \mathrm{H}_{2} \mathrm{SO}_{4}} \times 100} \\ {\% \text { of } \mathrm{S} \text { in } \mathrm{H}_{2} \mathrm{SO}_{4}=\frac{32}{98} \times 100}\end{array}\)
= 32.65 %.
Samacheer Kalvi 10th Science Atoms and Molecules Textual Evaluation Solved
I. Choose the best answer.
Question 1.
Which of the following has the smallest mass?
(a) 6.023 × 1023 atoms of He
(b) 1 atom of He
(c) 2 g of He
(d) 1 mole atoms of He.
Answer:
(b) 1 atom of He
Hint:
(a) 6.023 × 1023 atoms of He = 1 mole
Mass of 1 mole of He = 4 g (or) 0.004 kg.
(b) Mass of 1 atom of He =?
Mass of 6.023 × 1023 atoms of He = 0.004 kg.
Mass of 1 atom of He = \(\frac{0.004}{6.023 \times 10^{23}}=6.6423 \times 10^{-27} \mathrm{kg}\)
(c) 2 g of He = Mass = 0.002 kg.
(d) 1 mole atoms of He = 4 g = 0.004 kg.
So (b) is the smallest mass as 6.6423 × 10-27 kg.
Question 2.
Which of the following is a triatomic molecule?
(a) Glucose
(b) Helium
(c) Carbon dioxide
(d) Hydrogen
Answer:
(c) Carbon dioxide
Question 3.
The volume occupied by 4.4 g of CO2 at S.T.P _____.
(a) 22.4 litre
(b) 2.24 litre
(c) 0.24 litre
(d) 0.1 litre.
Answer:
(b) 2.24 litre
Hint:
Molar volume of CO2 = 22.4 litre.
The volume occupied by 1 mole.
i.e. 44 g (molar mass) of CO2.
44 g of CO2 occupied 22.4 litre of volume.
4.4 g of CO2 will occupy \(\frac{22.4}{44}\) × 4.4 = \(\frac{22.4}{10}\) = 2.24 litre.
So, answer (b) is correct.
Question 4.
Mass of 1 mole of Nitrogen atom is:
(a) 28 amu
(b) 14 amu
(c) 28 g
(d) 14 g
Answer:
(c) 28 g
![]()
Question 5.
Which of the following represents 1 amu?
(a) Mass of a C – 12 atom
(b) Mass of a hydrogen atom
(c) \(\frac { 1 }{ 2 }\)th of the mass of a C – 12 atom
(d) Mass of O – 16 atom
Answer:
(c) 1 / 12th of the mass of a C – 12 atom
Hint: By definition 1 amu is defined as precisely 1 / 12th the mass of an atom of carbon – 12.
So, answer (c) is correct.
Question 6.
Which of the following statement is incorrect?
(a) One gram of C – 12 contains Avogadro’s number of atoms.
(b) One mole of oxygen gas contains Avogadro’s number of molecules.
(c) One mole of hydrogen gas contains Avogadro’s number of atoms.
(d) One mole of electrons stands for 6.023 × 1023 electrons.
Answer:
(a) One gram of C – 12 contains Avogadro’s number of atoms.
Question 7.
The volume occupied by 1 mole of a diatomic gas at S.T.P is _____.
(a) 11.2 litre
(b) 5.6 litre
(c) 22.4 litre
(d) 44.8 litre.
Answer:
(c) 22.4 litre
Hint: By definition 1 mole of any gas at S.T.P occupies molar volume i.e. 22.4 litres.
So (c) is the correct answer.
Question 8.
In the nucleus of \(_{20} \mathrm{Ca}^{40}\), there are _____.
(a) 20 protons and 40 neutrons
(b) 20 protons and 20 neutrons
(c) 20 protons and 40 electrons
(d) 40 protons and 20 electrons.
Answer:
(b) 20 protons and 20 neutrons
Hint:
\(_{20} \mathrm{Ca}^{40}\)
20 = Atomic number = Number of protons (or) Number of electrons
40 = Mass number = Number of protons + Number of neutrons
\(_{20} \mathrm{Ca}^{40}\) contains 20 protons, 20 electrons and 20 neutrons.
So the answer (b) is correct.
Question 9.
The gram molecular mass of oxygen molecule is:
(a) 16 g
(b) 18 g
(c) 32 g
(d) 17 g
Answer:
(b) 18 g
Question 10.
1 mole of any substance contains ______ molecules.
(a) 6.023 × 1023
(b) 6.023 × 10-23
(c) 3.0115 × 1023
(d) 12.046 × 1023.
Answer:
(a) 6.023 × 1023
Hint:
Avogadro’s law states that 1 mole of any substance contains 6.023 × 1023 molecules.
So the answer (a) is correct.
II. Fill in the blanks
Question 1.
Atoms of different elements having ______ mass number, but ______ atomic numbers are called isobars.
Answer:
Same, different.
Question 2.
Atoms of different elements having the same number of _____ are called isotones.
Answer:
Neutrons.
Question 3.
Atoms of one element can be transmuted into atoms of other elements by _____.
Answer:
Artificial transmutation.
Question 4.
The sum of the numbers of protons and neutrons of an atom is called its _____.
Answer:
Mass number.
![]()
Question 5.
Relative atomic mass is otherwise known as _____.
Answer:
Standard atomic weight.
Question 6.
The average atomic mass of hydrogen is _____ amu.
Answer:
1.008 amu.
Question 7.
If a molecule is made of similar kind of atoms, then it is called ______ atomic molecule.
Answer:
Homo.
Question 8.
The number of atoms present in a molecule is called its _____.
Answer:
Atomicity.
Question 9.
One mole of any gas occupies _____ ml at S.T.P.
Answer:
22400.
Question 10.
Atomicity of phosphorous is _____.
Answer:
4.
III. Match the following.
Question 1.
| a. 8 g of O2 | i. 4 moles |
| b. 4 g of H2 | ii. 0.25 moles |
| c. 52 g of He | iii. 2 moles |
| d. 112 g of N2 | iv. 0.5 moles |
| e. 35.5 g of Cl2 | v. 13 moles |
Answer:
a – ii, b – iii, c – v, d – i, e – iv.
IV. True or False: (If false give the correct statement)
Question 1.
Two elements sometimes can form more than one compound.
Answer:
True.
Question 2.
Noble gases are Diatomic
Answer:
False.
Correct Statement: Noble gases are monoatomic
Question 3.
The gram atomic mass of an element has no unit?
Answer:
False.
Correct Statement: The gram atomic mass of an element is expressed in the unit grams.
Question 4.
1 mole of Gold and Silver contain the same number of atoms?
Answer:
True
Question 5.
The molar mass of CO2 is 42 g?
Answer:
False.
Correct Statement: The molar mass of CO2 is (12 + 32) = 44 g.
V. Assertion and Reason:
Answer the following questions using the data given below:
(i) A and R are correct, R explains the A.
(ii) A is correct, R is wrong.
(iii) A is wrong, R is correct.
(iv) A and R are correct, R doesn’t explain A.
Question 1.
Assertion: Atomic mass of aluminium is 27
Reason: An atom of aluminium is 27 times heavier than 1 / 12th of the mass of the C – 12 atoms.
Answer:
(i) A and R are correct, R explains the A.
Question 2.
Assertion: The Relative Molecular Mass of Chlorine is 35.5 a.m.u.
Reason: The natural abundance of Chlorine isotopes are not equal.
Answer:
(i) A and R are correct, R explains the A.
VI. Short Answer Questions
Question 1.
Define Relative atomic mass.
Answer:
Relative atomic mass of an element is the ratio between the average mass of its isotopes to 1 / 12th part of the mass of a carbon – 12 atom. It is denoted as Ar.
\(\text { Relative atomic mass }=\frac{\text { Average mass of the isotopes of the element }}{1 / 12^{\text {th }} \text { of the mass of one Carbon- } 12 \text { atom }}\).
Question 2.
Write the different types of isotopes of oxygen and its percentage abundance.
Answer:
Isotopes of oxygen:
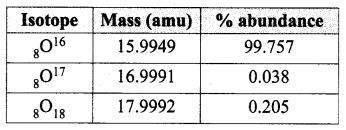
The atomic mass of oxygen = (15.9949 × 0.99757) + (16.9991 × 0.00038) + (17.9992 × 0.00205) = 15.999 amu.
Question 3.
Define Atomicity.
Answer:
The number of atoms present in the molecule is called atomicity.
Question 4.
Give any two examples for heteroatomic molecules.
Answer:
Heterodiatomic molecules.
e.g.,
- HCl
- NaCl.
Question 5.
What is Molar volume of a gas?
Answer:
The volume occupied by one mole of any gas at STP is called molar volume. Its value is equal to 22.4 litre or 22400 ml or 22400 cm³ or 2.24 × 10-2 m³.
Question 6.
Find the percentage of nitrogen in ammonia.
Answer:
Ammonia – NH3 = Molar mass = 14 + 3 = 17
Mass % of Nitrogen = \(\frac{14}{17} \times 100\) = 82.35%.
VII. Long Answer Questions.
Question 1.
Calculate the number of water molecule present in one drop of water which weighs 0.18 g.
Answer:
One mole of water weighs 18 g.
18 g of water contains 6.023 × 1023 water molecules.
∴ 0.18 g of water contains
= \(\frac{6.023×10^{23}}{18}\) × 0.18
= 6.023 × 1021 water molecules
Question 2.
N2 + 3H2 → 2NH3 (The atomic mass of nitrogen is 14, and that of hydrogen is 1)
- 1 mole of nitrogen (____ g) + ____.
- 3 moles of hydrogen (____ g) → ____.
- 2 moles of ammonia (____ g).
Answer:
N2 + 3H2 → 2NH3
- 1 mole of N2 = 28 g
- 3 moles of H2 = 6 g
- 2 moles of NH3 = 34 g
⇒ 1 mole of nitrogen (28 g) + 3 moles of hydrogen (6 g) → 2 moles of Ammonia (34 g).
Question 3.
Calculate the number of moles in:
(i) 27 g of Al
(ii) 1.51 × 1023 molecules of NH4Cl
Answer:
(i) 27 g of Al
Number of moles = \(\frac{\text { Mass }}{\text { Atomic mass }}\)
Number of moles in 27 g of Al = \(\frac{27}{27}\) = 1 mole.
(ii) 1.51 × 1023 molecules of NH4Cl
Number of moles = \(\frac{\text { Number of molecules }}{\text { Avogadro’s number }}=\frac{1.51 \times 10^{23}}{6.023 \times 10^{23}}=0.25 \text { moles. }\).
Question 4.
Give the salient features of ‘Modern atomic theory’.
Answer:
(i) An atom is no longer indivisible.
(ii) Atoms of the same element may have different atomic mass.
Eg: isotopes 17Cl35, 17Cl37.
(ii) Atoms of different elements may have same atomic masses.
Eg: Isobars 18Ar40, 20Ca40.
(iv) Atoms of one element can be transmuted into atoms of other elements. An atom is no longer indestructible.
(v) Atoms may not always combine in a simple whole number ratio.
Eg: Glucose C6H12O6 C : H : O = 6 : 12 : 6 or 1 : 2 : 1.
(vi) Atom is the smallest particle that takes part in a chemical reaction.
(vii) The mass of an atom can be converted into energy (E = mc²).
Question 5.
Derive the relationship between Relative molecular mass and Vapour density.
Answer:
(i) The Relative Molecular Mass of a gas or vapour is the ratio between the mass of one molecule of the gas or vapour to mass of one atom of Hydrogen.
(ii) Vapour density is the ratio of the mass of a certain volume of a gas or vapour, to the mass of an equal volume of hydrogen, measured under the same conditions of temperature and pressure.
Vapour Density (V.D.) = \(\frac{\text { Mass of a given volume of gas or vapour at S.T.P }}{\text { Mass of same volume of hydrogen }}\).
(iii) According to Avogadro’s law, equal volumes of all gases contain equal number of molecules. Thus, let the number of molecules in one volume = n, then
(iv) V.D at STP = \(\frac{\text { Mass of “n’molecules of a gas or vapour at S.T.P }}{\text { Mass of ‘n’molecules of hydrogen }}\)
Cancelling ‘n’ which is common, you get
V.D = \(\frac{\text { Mass of } 1 \text { molecule of a gas or vapour at S.T.P. }}{\text { Mass of } 1 \text { molecules of hydrogen }}\).
(v) Since hydrogen is diatomic
\(\mathrm{V} . \mathrm{D} .=\frac{\text { Mass of } 1 \text { molecule of a gas or vapour at S.T.P. }}{\text { Mass of } 2 \text { atoms of hydrogen }}\).
(vi) By comparing the definition of relative molecular mass and vapour density we can write as follows.
\(\mathrm{V.D.}=\frac{\text { Mass of } 1 \text { molecule of a gas or vapour at S.T.P. }}{2 \times \text { Mass of } 1 \text { atom of hydrogen }}\)
Relative molecular mass (hydrogen scale) \(=\frac{\text { Mass of } 1 \text { molecule of a gas or vapour at STP. }}{\text { Mass of } 1 \text { atom of hydrogen }}\).
(vii) By substituting the relative molecular mass value in vapour density definition, we get
Vapour density (V.D.) = Relative molecular mass / 2
⇒ 2 × vapour density = Relative molecular mass of a gas.
VIII. HOT Questions
Question 1.
Calcium carbonate is decomposed on heating in the following reaction CaCO3 → CaO + CO2
(i) How many moles of Calcium carbonate are involved in this reaction?
Answer:
One mole
(ii) Calculate the gram molecular mass of calcium carbonate involved in this reaction.
Answer:
Gram molecular mass of CaCO3
= 40 + 12 + 3(16)
= 100 g
(iii) How many moles of CO2 are there in this equation?
Answer:
One mole.
![]()
IX. Solve the following problems.
Question 1.
How many grams are there in the following?
Answer:
Formula = No. of moles (n) × (Gram molecular mass)
(i) 2 moles of hydrogen molecule, H2
Answer:
Mass of 2 moles of H2 molecule
= 2 × 2 = 4 g
(ii) 3 moles of chlorine molecule, Cl2
Answer:
Gram molecular mass of 3 moles of Cl2
= 3 × 71 = 213 g
(iii) 5 moles of sulphur molecule, S2
Answer:
Gram molecular mass of 5 moles of S2
= 5 × 8(32)
= 5 × 256 = 1280 g
(iv) 4 moles of phosphorous molecule, P4
Answer:
Gram molecular mass of 4 moles of P2
= 4 × 4(31)
= 4 × 124 = 496 g
Question 2.
Calculate the % of each element in calcium carbonate. (Atomic mass: C – 12, O – 16, Ca – 40)
Solution:
Calcium carbonate: CaCO3
Molar mass of CaCO3 = 40 + 12 + (16 × 3) = 100 g
% of Calcium \(=\frac{40}{100} \times 100=40 \%\)
% of Carbon \(=\frac{12}{100} \times 100=12 \%\)
% of Oxygen \(=\frac{48}{100} \times 100=48 \%\).
Question 3.
Calculate the % of oxygen in Al2(SO4)3. (Atomic mass: Al – 12, O – 16, S – 32)
Solution:
Aluminium Sulphate – Al2(SO4)3
Molar mass of Aluminium Sulphate = (27 × 2) + (32 × 3) + (16 × 12) = 54 + 96 + 192 = 342 g
% of Oxygen \(=\frac{192}{342} \times 100=56.14 \%\).
Question 4.
Calculate the % relative abundance of B – 10 and B – 11, if its average atomic mass is 10.804 amu.
Solution:
The average atomic mass of Boron = 10.804 amu.
% relative abundance of B – 10 = ?
% relative abundance of B – 11 = ?
Let the fraction of relative abundance of B – 10 = x
Let the fraction of relative abundance of B – 11 = y
x + y = 1
y = 1 – x
Relative abundance = x (10) + (1 – x) (11) = 10.804 amu
⇒ 10x + 11 – 11x = 10.804 amu
⇒ 11 – x = 10.804 amu
⇒ -x = 10.804 – 11
⇒ -x = -0.196
⇒ x = 0.196
x = % abundance of B – 10 = 0.196 × 100 = 19.6 %
y = % abundance of B – 11 = 100 – 19.6 = 80.4 %
Percentage abundance of B – 10 = 19.6 %
Percentage abundance of B – 11 = 80.4 %.
Activities
Question 1.
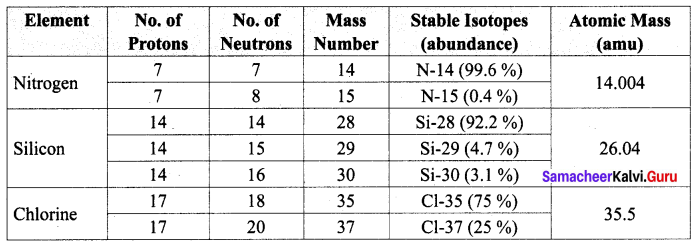
Complete the following table by filling the appropriate values / terms
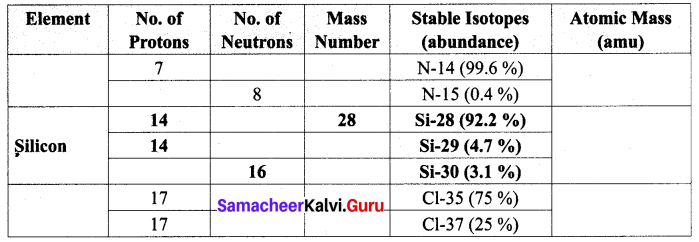
Solution:
Question 2.
Classify the following molecules based on their atomicity and fill in the table:
Fluorine (F2), Carbon dioxide (CO2), Phosphorous (P4), Sulphur (S8), Ammonia (NH3), Hydrogen iodide (HI), Sulphuric Acid (H2SO4), Methane (CH4), Glucose (C6H12O6), Carbon monoxide (CO)

Solution:
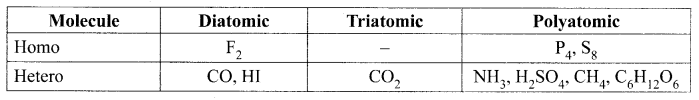
Question 3.
Under same conditions of temperature and pressure if you collect 3 litres of O2, 5 litres of Cl2 and 6 litres of H2,
- Which has the highest number of molecules?
- Which has the lowest number of molecules?
Solution:
Number of moles of O2 \(=\frac{\text { Volume at S.T.P }}{\text { Molar volume }}\) \(=\frac{3}{22.4}\) = 0.1339 moles
Number of molecules = Number of moles × Avogadro number
= 0.1339 × 6.023 × 1023
= 0.8064 × 1023
= 8.064 × 1022 O2 molecules.
Number of moles of Cl2 = \(\frac{5}{22.4}\) = 0.2232 moles
Number of molecules = 0.2232 × 6.023 × 1023 = 1.344 × 1023 molecules.
Number of moles of H2 = \(\frac{6}{22.4}\) = 0.2678 moles
Number of molecules = 0.2678 × 6.023 × 1023 = 1.6129 × 1023 molecules.
- 6 litres of H2 has the highest number of molecules.
- 3 litres of O2 has the lowest number of molecules.
Samacheer Kalvi 10th Science Atoms and Molecules Additional Question Solved
I. Choose the best answer.
Question 1.
Which of the following pair indicates isotopes?
(a) \(_{17} \mathrm{Cl}^{35},_{17} \mathrm{Cl}^{37}\)
(b) \(_{18} \mathrm{Ar}^{40},_{20} \mathrm{Ca}^{40}\)
(c) \(_{6} \mathrm{C}^{13},_{7} \mathrm{N}^{14}\)
(d) \(_{33} \mathrm{As}^{77},_{34} \mathrm{Se}^{78}\).
Answer:
(a) \(_{17} \mathrm{Cl}^{35},_{17} \mathrm{Cl}^{37}\)
Question 2.
The mass of a proton is equal to:
(a) 1 amu
(b) \(\frac{1}{12^{th}}\) of the mass of a C – 12 atom
(c) zero
(d) both (a) and (b)
Answer:
(d) both (a) and (b)
Question 3.
The sum of the number of protons and neutrons of an atom is called its _____.
(a) nucleus
(b) atomic number
(c) mass number
(d) relative atomic mass.
Answer:
(c) mass number
Question 4.
Total number of electrons present in 1.7 g of NH3 is:
(a) 6.023 × 1023
(b) 6.023 × 1024
(c) 6.023 × 1022
(d) 6.023 × 1025
Answer:
(a) 6.023 × 1023
Question 5.
An isotope of hydrogen without neutrons is _____.
(a) Deuterium \(_{1} \mathrm{H}^{2}\)
(b) Protium \(_{1} \mathrm{H}^{1}\)
(c) Tritium \(_{1} \mathrm{T}^{3}\)
(d) Heavy hydrogen \(_{1} \mathrm{D}^{2}\).
Answer:
(b) Protium \(_{1} \mathrm{H}^{1}\)
![]()
Question 6.
The isotope tritium contains 1 proton and neutron in the nucleus.
(a) 1
(b) 2
(c) 3
(d) none
Answer:
(b) 2
Question 7.
Which one of the following element is used as the standard for measuring the relative atomic mass of an element in now a days?
(a) \(_{1} \mathrm{H}^{2}\)
(b) \(6^{\mathrm{O}^{12}}\)
(c) C – 12
(d) C – 14.
Answer:
(c) C – 12
Question 8.
The atom with no neutrons in the nucleus is:
(a) He
(b) Deuterium
(c) Tritium
(d) Protium
Answer:
(d) Protium
Question 9.
The average atomic mass of carbon is _____.
(a) 12 amu
(b) 12.84 amu
(c) 24.011 amu
(d) 12.011 amu.
Answer:
(d) 12.011 amu.
Question 10.
Which one of the following is the most abundant element in both the Earth’s crust and in the human body?
(a) Carbon
(b) Silicon
(c) Oxygen
(d) Hydrogen.
Answer:
(c) Oxygen
Question 11.
Gram molecular mass of H2SO4 is:
(a) 49 g
(b) 54 g
(c) 98 g
(d) 100 g
Answer:
(c) 98 g
Question 12.
Boron – 10 and Boron – 11 are called _____.
(а) isotopes
(b) isobars
(c) isotones
(d) isomers.
Answer:
(c) isotopes
Question 13.
Which of the following are found in the elementary state in nature?
(a) Hydrogen chloride
(b) Carbon dioxide
(c) Noble gases
(d) Oxygen.
Answer:
(c) Noble gases
Question 14.
Ammonia gas is formed by the following reaction
N2(g) + 3H2(g) → 2NH3(g)
The volume of H2 required to form 6 dm3 of NH3 is:
(a) 9 dm³
(b) 10 dm³
(c) 4 dm³
(d) 2 dm³
Answer:
(a) 9 dm³
![]()
Question 15.
Which one of the following is a hetero diatomic molecule?
(a) O2
(6) N2
(c) HI
(d) CH4.
Answer:
(c) HI
Question 16.
Which one of the following is a hetero triatomic molecule?
(a) H2O
(b) BCl3
(c) CH4
(d) PCl5.
Answer:
(a) H2O
Question 17.
1 gm atom of nitrogen represents:
(a) 6.023 × 102 N2 molecules
(b) 22.4 litre of N2 at STP
(c) 11.2 L of N2 at STP
(d) 28 g of nitrogen
Answer:
(c) 11.2 L of N2 at STP
Question 18.
Find out the hetero diatomic molecule?
(a) Hydrogen
(b) Hydrogen chloride
(c) Methane
(d) Ammonia.
Answer:
(b) Hydrogen chloride
Question 19.
Which one of the following is an example of a polyatomic molecule?
(a) Sulphur
(b) Gold
(c) Sodium
(d) Helium.
Answer:
(a) Sulphur
Question 20.
The gram molar mass of CO2 is:
(a) 44 g
(b) 100 g
(c) 4.4 g
(d) 22 g
Answer:
(a) 44 g
Question 21.
Which one of the following is an example of a polyatomic molecule?
(a) Fluorine
(b) Glucose
(c) Oxygen
(d) Sodium.
Answer:
(b) Glucose (C6H12O6)
Question 22.
The gram molecular mass of water is _____.
(a) 18 amu
(b) 18 g
(c) 18 u
(d) 18.
Answer:
(b) 18 g
Question 23.
The value of Avogadro’s number is _____.
(a) 6.023 × 10-23
(b) 6.023 × 1023
(c) 22.4
(d) 22400.
Answer:
(b) 6.023 × 1023
Question 24.
The value of molar volume is _____.
(a) 22.4 ml
(b) 22.4 litres
(c) 22400 litres
(d) 2.24 litres.
Answer:
(b) 22.4 litres
Question 25.
Which one of the following represent Avogadro’s law?
(a) V ∝ \(\frac{1}{n}\)
(b) V ∝ n
(c) V ∝ \(\frac{1}{n^{2}}\)
(d) V2 ∝ \(\frac{1}{n}\).
Answer:
(b) V ∝ n
![]()
Question 26.
Which of the following has the highest number of molecules?
(a) 1 litre of N2
(b) 2 litres of oxygen
(c) 5 litres of Cl2
(d) 6 litres of Hydrogen.
Answer:
(d) 6 litres of Hydrogen.
Question 27.
Which one of the following has the lowest number of molecules?
(a) 1 litre of N2
(b) 2 litres of H2
(c) 3 litres of O2
(d) 4 litres of Cl2
Answer:
(a) 1 litre of N2
Question 28.
2 × Vapour density is equal to _____.
(a) atomic mass
(b) valency
(c) relative molecular mass
(d) atomic number.
Answer:
(c) relative molecular mass
Question 29.
The value of gram molar mass of CO2 is _____.
(a) 44 amu
(b) 44 g
(c) 44
(d) 44 kg.
Answer:
(b) 44 g
Hint: Molar mass = 12 + (16 × 2) = 44 g.
Question 30.
The number of moles of a sample that contain 36 g of water is _____.
(a) 1 mole
(b) 0.5 mole
(c) 4 moles
(d) 2 moles.
Answer:
(d) 2 moles
Hint: 18 g of water = 1 mole
36 g of water = \(\frac{1}{18} \times 36\) = 2 moles
Question 31.
Which of the following has the largest number of particles?
(a) 8 g of CH4
(b) 4.4 g of CO2
(c) 34.2 g of C12H22O11
(d) 2 g of H2.
Answer:
(d) 2 g of H2.
Hint. 2 g = Molar mass = 1 mole = 6.023 × 1023 particles.
Others are less.
Question 32.
The number of molecules in 16.0 g of oxygen is _____.
(a) 6.023 × 1023
(b) 6.023 × 10-23
(c) 3.01 × 10-23
(d) 3.011 × 1023
Answer:
(d) 3.011 × 1023
Hint: 32 g of oxygen contain 6.023 × 1023 molecules.
16 g of oxygen will contain
\(\frac{6.023 \times 10^{23}}{32} \times 16=3.011 \times 10^{23}\)
Question 33.
The percentage of hydrogen in H2O is _____.
(a) 8.88
(b) 11.2
(c) 20.60
(d) 80.0.
Answer:
(b) 11.2
Hint: 1 mole of H2O has 2.016 g of H2
Percentage of H2 = \(\frac{2.016}{18}\) × 100 = 11.2
Question 34.
Which of the following contains the largest number of molecules?
(a) 0.2 mole of H2
(b) 8.0 g of H2
(c) 17 g of H2O
(d) 6.0 g of CO2
Answer:
(b) 8.0 g of H2
Hint: No. of moles = \(\frac{8}{2}\) = 4 moles.
No. of molecules = mole × Avogadro number = 4 × 6.023 × 1023 = 2.409 × 1024
Question 35.
One gram of which of the following contains the largest number of oxygen atoms?
(a) O
(b) O2
(c) O3
(d) All contain the same
Answer:
(c) O3
![]()
Question 36.
The percentage by weight of O2 in CaSO4. (O = 16, Ca = 40, S = 32) is _____.
(a) 64 %
(b) 28.2 %
(c) 47.05 %
(d) 16.2 %.
Answer:
(c) 47.05 %
Hint: % by weight of O2 = \(\frac{64}{136}\) × 100 = 47.05 %.
Question 37.
One mole of a gas occupies a volume of 22.4 L. This is derived from _____.
(a) Berzilliu’s hypothesis
(b) Gay – Lussac’s law
(c) Avogadro’s law
(d) Dalton’s law.
Answer:
(c) Avogadro’s law
Question 38.
Volume of gas at STP is 1.12 × 10-7 cc. Calculate the number of molecules in it.
(a) 3.011 × 1020
(b) 3.011 × 1012
(c) 3.011 × 1023
(d) 3.011 × 1024
Answer:
(b) 3.011 × 1012
Hint. 2.24 × 10-3 c molecules 6.023 × 1023 molecules
1.12 × 10-7 cc contains = \(\frac{6.023 \times 10^{23}}{22400} \times 1.12 \times 10^{-7}\)
= 3.011 × 1012.
Question 39.
The number of molecules of CO2 present in 44 g of CO2 is _____.
(a) 6.023 × 1023
(b) 3.011 × 1023
(c) 12 × 1023
(d) 3 × 1010.
Answer:
(a) 6.023 × 1023 (Avogadro number).
Question 40.
The volume occupied by 4.4 g of CO2 at S.T.P is _____.
(a) 22.4 L
(b) 2.24 L
(c) 0.224 L
(d) 0.1 L.
Answer:
(b) 2.24 L
Hint. 44 g of CO2 at S.T.P occupies 22.4 L
4.4 g of CO2 at S.T.P will occupy \(\frac{22.4}{44}\) × 4.4 = 2.24 L.
Question 41.
How many molecules at present in one gram of hydrogen?
(a) 6.023 × 1023
(b) 3.011 × 1023
(c) 2.5 × 1023
(d) 1.5 × 1023
Answer:
(b) 3.011 × 1023
Hint: H2 = Molar mass = 2 g
2 g of H2 contains 6.023 × 1023 molecules
∴ 1 g of H2 will contain = \(\frac{6.023 \times 10^{23}}{2} \times 1\)
= 3.011 × 1023 molecules.
Question 42.
Atoms which have the same number of protons but different number of neutrons are called _____.
(a) isotopes
(b) isomers
(c) allotropes
(d) isotones.
Answer:
(a) isotopes
Question 43.
Number of atoms which a molecule to sulphur contains is _____.
(a) 3
(b) 8
(c) 4
(d) 2.
Answer:
(b) 8 (S8)
Question 44.
An example of a triatomic molecule is _____.
(a) Ozone
(b) Nitrogen
(c) Hydrogen
(d) Ammonia.
Answer:
(a) Ozone
Question 45.
The atomic mass of sodium is 23. The number of moles in 46 g of sodium is _____.
(a) 0.5
(b) 2
(c) 1
(d) 0.25.
Answer:
(b) 2
Hint:
No. of moles = \(\frac{\text { Mass }}{\text { Atomic mass }}=\frac{46}{23}\) = 2.
Question 46.
The number of atoms in a molecule of the elementary substance is called _____.
(a) Atomic number
(b) Avogadro number
(c) Atomic mass
(d) Atomicity.
Answer:
(d) Atomicity.
Question 47.
Avogadro number represents the number of atoms in _____.
(a) 12 g of C – 12
(b) 4.4 g of CO2
(c) 320 g of Sulphur
(d) 1 g of C – 12
Answer:
(a) 12 g of C – 12
Question 48.
The number of moles in 5 grams of Calcium is _____.
(a) 0.5 mole
(b) 0.125 mole
(c) 1.25 mole
(d) 12.5 mole.
Answer:
(a) 0.125 mole
Hint:
No. of moles = \(\frac{\text { Mass }}{\text { Atomic mass }}\)
\(=\frac{5}{40}=\frac{1}{8}\) = 0.125 mole.
Question 49.
One mole of H2O corresponds to _____.
(a) 22.4 litre at 1 atm and 250°C
(b) 6.023 × 1023 atoms of hydrogen and 6.023 × 1023 atoms of oxygen
(c) 18 g
(d) 1 g.
Answer:
(c) 18 g
Hint: One mole = Molar mass = 2 + 16 = 18 g.
Question 50.
Which one of the following has the maximum number of atoms?
(a) 18 g of H2O
(b) 18 g of O2
(c) 18 g of CO2
(d) 18 g of CH4.
Answer:
(d) 18 g of CH4.
Hint:
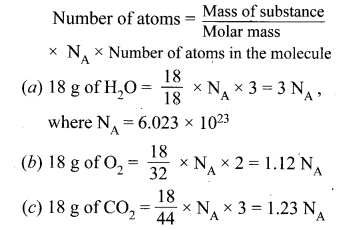
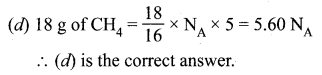
Question 51.
The atomicity of K2Cr2O7 is _____.
(a) 9
(b) 11
(c) 10
(d) 12.
Answer:
(b) 11
Question 52.
All noble gases are _____ molecules.
(a) diatomic
(b) triatomic
(c) mono atomic
(d) poly atomic.
Answer:
(c) mono atomic
Question 53.
The total number of atoms represented by the compound CuSO4 . 5H2O is ____.
(a) 27
(b) 21
(c) 5
(d) 8.
Answer:
(b) 21
Question 54.
Which one of the following represents the mass of 0.5 moles of water molecules?
(a) 18 g
(b) 1.8 g
(c) 9 g
(d) 4.5 g.
Answer:
(c) 9 g
\(\text { Mole }=\frac{\text { Mass }}{\text { Molecular mass }}\)
Mass = Mole × Molecular mass = 0.5 × 18 = 9 g.
Question 55.
The atomic mass of Calcium is 40. Calculate the number of moles in 16 g of Calcium.
(a) 0.4 mole
(b) 4 moles
(c) 640 moles
(d) \(\frac { 1 }{ 4 }\) mole.
Answer:
(a) 0.4 mole
Hint:
\(\text { Mole }=\frac{\text { Mass }}{\text { Atomic mass }}=\frac{16}{40}=\frac{8{}}{20}\) \(=\frac{4}{10}=0.4 \mathrm{mole}\).
Question 56.
If the atomic mass of sodium is 23 amu, then the mass of 3.011 × 1023 sodium atoms is _____.
(a) 11.5 kg
(b) 23 g
(c) 0.5 mole
(d) 11.5 g.
Answer:
(d) 11.5 g.
Hint: Mass of 6.023 × 1023 sodium atoms = 23 amu = 23 g.
∴ Mass of 3.011 × 1023 sodium atoms
\(=\frac{23}{6.023 \times 10^{23}} \times 3.011 \times 10^{23}=11.5 \mathrm{g}\).
Question 57.
Which of the following will have maximum mass?
(а) 0.1 mole of NH2
(b) 1022 atoms of carbon
(c) 1022 molecules of CO2
(d) 1 g of Fe
Answer:
(a) 0.1 mole of NH3
Hint:
(a) 0.1 mole of NH3 has 6.023 × 1023 atoms.
Mass of 1 mole of NH3 = 17 g
Mass of 0.1 mole of NH3 = 1.7 g.
(b) Mass of 1022 atoms of carbon
6.023 × 1023 c atoms mass = 12 g
1022 atoms of C has the mass
\(=\frac{12}{6.023 \times 10^{23}} \times 1022=2.036 \times 10^{-20} \mathrm{g}\).
(c) Mass of 1022 molecules of CO2
CO2 = molar mass = 44 g
6.023 × 1023 CO2 molecules has the mass = 44 g
∴ 1022 CO2 molecules has the mass 44
\(=\frac{44}{6.023 \times 10^{23}} \times 1022=7.466 \times 10^{-20} \mathrm{g}\).
(d) 1 g of Fe
∴ (a) 1.7 g of NH3 has the highest mass.
Question 58.
Which of the following correctly represents 360 g of water?
(i) 2 moles of water
(ii) 20 moles of water
(iii) 6.023 × 1023 molecules of water
(iv) 1.2044 × 1025 molecules of water
(a) (i) only
(b) (i) and (iv)
(c) (ii) and (iii)
(d) (ii) and (iv).
Answer:
(d) (ii) and (iv).
Hint: (i) 2 moles of water
Mass of 1 mole of water = 18 g
Mass of 2 moles of water = 18 × 2 = 36 g.
(ii) 20 moles of water
Mass of 1 mole of water = 18 g
Mass of 20 moles of water = 18 × 20 = 360 g.
(iii) 6.023 × 1023 molecules of water = 1 mole = 18 g.
(iv) 1.2044 × 1025 molecules of water
6.23 × 1023 molecules of water = 1 mole
∴ 1.2044 × 1025 molecules
\(=\frac{1}{6.023 \times 10^{23}} \times 1.2044 \times 10^{25}\)
= 20 moles.
∴ Mass of 20 moles = 20 × 18 = 360 g.
So (d) is correct.
Question 59.
Which of the following contains maximum number of molecules?
(a) 1 g of CO2
(b) 1 g of N2
(c) 1 g of H2
(d) 1 g of CH4
Answer:
(b) 1 g of H2
Hint:
(a) 1 g of CO2
No. of moles = \(\frac{\text { Mass }}{\text { Atomic mass }}\) (or) \(\frac{\text { Mass }}{\text { Molecular mass }}\)
No. of moles of 1 g of CO2 = \(\frac{1}{44}\)
No. of molecules = \(\frac{1}{44}\) × 6.023 × 1023
= 1.368 × 1022 molecules of CO2.
(b) 1 g of N2
No. of molecules = \(\frac{1}{28}\) × 6.023 × 1023
= 2.151 × 1022 molecules of N2.
(c) 1 g of H2
No. of molecules = \(\frac{6.023 \times 10^{23} \times 1}{2}\)
= 3.011 × 1023 molecules of H2
(d) 1 g of CH4
No. of molecules = \(\frac{6.023 \times 10^{23} \times 1}{16}\)
= 3.764 × 1022 molecules of CH4
So (c) is the correct answer.
Question 60.
Which of the following pair is an example of isotopes?
\(\begin{array}{l}{\text { (a) } 21 \mathrm{Sc}^{45} \text { and }_{23} \mathrm{V}^{50}} \\ {\text { (b) }_{22} \mathrm{Ti}^{48} \text { and }_{22} \mathrm{Ti}^{50}} \\ {\text { (c) }_{22} \mathrm{Ti}^{50} \text { and }_{23} \mathrm{V}^{50}} \\ {\text { (d) }_{21} \mathrm{Sc}^{45} \text { and }_{22} \mathrm{Ti}^{50}}\end{array}\)
Answer:
(b) \(\text { (b) }_{22} \mathrm{Ti}^{48} \text { and }_{22} \mathrm{Ti}^{50}\).
![]()
II. Fill in the blanks.
Question 1.
Amedeo Avogadro put forward a hypothesis based on the relation between the number of _____ and the _____ of gases.
Answer:
Molecules, volume.
Question 2.
The molar volume of a gas at STP is _____ and the value of Avogadro Number is _____.
Answer:.
22.4.litres, 6.023 x 1023.
Question 3.
Nitrogen and oxygen are _____ molecules whereas Helium and Neon are ____ molecules.
Answer:
Diatomic, monoatomic
Question 4.
- _____ are the building blocks of matter.
- ______ is a triatomic molecule.
Answer:
- Atoms and molecules
- Ozone
Question 5.
NH3, H2O are _____ molecules whereas N2, O2 are _____ molecules.
Answer:
Heteroatomic, Homoatomic
Question 6.
____ and ____ are polyatomic molecules.
Answer:
Phosphorous (P4), Sulphur (S8)
Question 7.
- Atoms of the same element with same atomic number but a different mass number are called _____.
- Atoms of different elements with the same number of neutrons are called _____.
Answer:
- Isotopes
- Isotones
Question 8.
Atomicity of Nitrogen is _____ whereas the atomicity of Helium is _____.
Answer:
2, 1.
Question 9.
Atoms of the same element with same atomic number but having different mass number are called _____.
Answer:
Isotopes.
Question 10.
Atoms of different elements with the same atomic mass but a different atomic number are called _____.
Answer:
Isobars.
Question 11.
Atoms of different elements having the same number of neutrons but a different atomic number and different mass number are called _____.
Answer:
Isotones.
Question 12.
_____ is the smallest particle that takes part in the chemical reaction.
Answer:
Atom.
![]()
Question 13.
Anything that has mass and occupies space is called _____.
Answer:
Matter.
Question 14.
Protons and neutrons have considerable mass, but _____ don’t have considerable mass.
Answer:
Atoms.
Question 15.
_____ is one-twelfth of the mass of C – 12 atom, an isotope of carbon which contains _____ protons and ____ neutrons.
Answer:
The atomic mass unit, 6, 6.
Question 16.
_____ are the building blocks of matter.
Answer:
Atoms.
Question 17.
The stable isotope of _____ is used as the standard for measuring the relative atomic mass of an element.
Answer:
Carbon C – 12.
Question 18.
Modem methods of determination of atomic mass by _____ use C – 12 as standard.
Answer:
Mass Spectrometry.
Question 19.
The relative atomic mass of sulphur is _____.
Answer:
32.
Question 20.
The average atomic mass of carbon is ______.
Answer:
12.011 amu.
Question 21.
The average atomic mass of an element becomes fractional due to the presence of ______.
Answer:
Isotopes.
Question 22.
_____ is the most abundant element in both the Earth’s crust and the human body.
Answer:
Oxygen.
Question 23.
Except for _____ atoms of most of the elements are found in the combined form with itself or atoms of other elements.
Answer:
Noble gases.
Question 24.
A molecule is a combination of two or more atoms held together by _____.
Answer:
Chemical bonds.
Question 25.
If the molecule is made of similar kind of atoms, it is called ______.
Answer:
Homo atomic molecule.
![]()
Question 26.
The molecule that consists of atoms of different elements is called _____.
Answer:
Hetero atomic molecule.
Question 27.
The number of _____ present in the molecule is called its atomicity.
Answer:
atoms.
Question 28.
The atomicity of ozone is _____.
Answer:
3.
Question 29.
The atomicity of hydrogen chloride is _____.
Answer:
2.
Question 30.
Water is a _____ molecule.
Answer:
Hetero triatomic.
Question 31.
One mole of an element contains ______ atoms and it is equal to its gram atomic mass.
Answer:
6.023 × 1023
Question 32.
One mole of any gas occupies ______ or _____ at S.T.P.
Answer:
22.4 litre, 22400 ml.
Question 33.
The _____ is useful to determine the empirical formula and molecular formula.
Answer:
Percentage composition.
Question 34.
The percentage composition of elements is useful to determine _____ and _____.
Answer:
Empirical formula, molecular formula.
Question 35.
Avogadro’s law is in agreement with ______.
Answer:
Dalton’s atomic theory.
Question 36.
_____ determines the relation between molecular mass and vapour density.
Answer:
Avogadro’s law.
Question 37.
Relative molecular mass is equal to _____.
Answer:
2 × Vapour density.
Question 38.
Atomicity of sulphur is _____.
Answer:
8.
Question 39.
The metals Cu, Ag, Au are _____ elements.
Answer:
Monoatomic.
![]()
Question 40.
The atomicity of H2SO4 is ______.
Answer:
7.
Question 41.
Atomicity of an element is equal to _____.
Answer:
\(\frac{\text { Molecular mass }}{\text { Atomic mass }}\)
III. Match the following.
Question 1.
| i. Monoatomic molecule | (a) Ozone |
| ii. Diatomic molecule | (b) Phosphorous |
| iii. Triatomic molecule | (c) Helium |
| iv. Polyatomic molecule | (d) Oxygen |
Answer:
i – c, ii – d, iii – a, iv – b.
Question 2.
| i. 22.4 litres | (a) Avogadro Number |
| ii. 6.023 × 1023 | (b) Molar volume |
| iii. 2 × vapour density | (c) 1 mole |
| iv. Mass / Atomic mass | (d) Molecular mass |
Answer:
i – b, ii – a, iii – d, iv – c.
Question 3.
| i. \(_{17} \mathrm{Cl}^{35},_{17} \mathrm{Cl}^{37}\) | (a) Isotones |
| ii. \(_{6} \mathrm{Cl}^{13},_{7} \mathrm{N}^{14}\) | (b) Isobars |
| iii. \(_{18} \mathrm{Ar}^{40},_{20} \mathrm{Ca}^{40}\) | (c) E = mc2 |
| iv. Einstein’s equation | (d) Isotopes |
Answer:
i – d, ii – a, iii – b, iv – c.
Question 4.
| i. H2O | (a) 180 g |
| ii. NH3 | (b) 44 g |
| iii. CO2 | (c) 17 g |
| iv. C6H12O6 | (d) 18 g |
Answer:
i – d, ii – c, iii – b, iv – a.
Question 5.
| i. NH3, CH4 | (a) Polyatomic molecule |
| ii. O2, N2 | (b) Monoatomic molecule |
| iii. He, Ne | (c) Heteroatomic molecule |
| iv. Sulphur | (d) Diatomic molecule |
Answer:
i – c, ii – d, iii – b, iv – a.
Question 6.
| i. F2 | (a) Polyatomic molecule |
| ii. O3 | (b) Monoatomic molecule |
| iii. P4 | (c) Diatomic molecule |
| iv. He | (d) Triatomic molecule |
Answer:
i – c, ii – d, iii – a, iv – b.
Question 7.
| i. H2 | (a) Hetero diatomic molecule |
| ii. HCl | (b) Monoatomic molecule |
| iii. H2O | (c) Homo diatomic molecule |
| iv. Ne | (d) Hetero triatomic molecule |
Answer:
i – c, ii – a, iii – d, iv – b.
Question 8.
| i. Isotopes | (a) S8, P4 |
| ii. Isobars | (b) \(_{6} \mathrm{C}^{13},_{7} \mathrm{N}^{14}\) |
| iii. Isotones | (c) \(_{1} \mathrm{H}^{1},_{1} \mathrm{H}^{2},_{1} \mathrm{H}^{3}\) |
| iv. Polyatomic molecule | (d) \(_{18} \mathrm{Ar}^{40},_{20} \mathrm{Ca}^{40}\) |
Answer:
i – c, ii – d, iii – b, iv – a.
Question 9.
| i. H2O | (a) 16 |
| ii. CO2 | (b) 18 |
| iii. C6H12O6 | (c) 44 |
| iv. CH4 | (d) 180 |
Answer:
i – b, ii – c, iii – d, iv – a
Question 10.
| i. 22 g of CO2 | (a) 2 moles |
| ii. 18 g of H2O | (b) 4 moles |
| iii. 360 g of Glucose | (c) 0.5 mole |
| iv. 64 g of CH4 | (d) 1 mole |
Answer:
i – c, ii – d, iii – a, iv – b.
IV. State whether true or false. If false, give the correct statement.
Question 1.
Isotopes are the atoms of the same element may not be similar in all respects.
Answer:
True.
Question 2.
Isobars are the atoms of the different elements with the same atomic number and different mass numbers.
Answer:
False.
Correct statement: Isobars are the atoms of the different elements with the same mass number but a different atomic number.
Question 3.
Isotones are the atoms of different elements with the same number of neutrons.
Answer:
True.
Question 4.
The number of molecules present in one mole of an element is called atomicity of an element.
Answer:
False.
Correct statement: The number of atoms present in one molecule of an element is called the atomicity of an element.
![]()
Question 5.
Avogadro’s hypothesis is used in the deduction of atomicity of elementary gases.
Answer:
True.
Question 6.
The volume of a gas at a given temperature and pressure is proportional to the number of particles.
Answer:
True.
Question 7.
The value of Gram molar volume at STP is 11.2 litres.
Answer:
False.
Correct statement: The value of Gram molar volume at STP is 22.4 litres.
Question 8.
The atomicity of nitrogen, oxygen and hydrogen is two.
Answer:
True.
Question 9.
Atoms and molecules are the building blocks of matter.
Answer:
True.
Question 10.
The atoms of certain elements such as hydrogen, oxygen and nitrogen have an independent existence.
Answer:
False.
Correct statement: The atoms of certain elements such as hydrogen, oxygen and nitrogen do not have an independent existence.
Question 11.
A molecule is the simplest structural unit of an element or compound which contains one or more atoms.
Answer:
True.
Question 12.
Phosphorous and sulphur are monoatomic molecules.
Answer:
False.
Correct statement: Phosphorous and sulphur are polyatomic molecules.
Question 13.
H2O, NH3, CH4 are examples of homoatomic molecules.
Answer:
False.
Correct statement: H2O, NH3, CH4 are examples of heteroatomic molecules.
![]()
Question 14.
An atom of one element can be transmuted into an atom of other element is known as artificial transmutation.
Answer:
True.
Question 15.
The molecule is the smallest particle that takes part in a chemical reaction.
Answer:
False.
Correct statement: Atom is the smallest particle that takes part in a chemical reaction.
Question 16.
The sum of the number of protons and neutrons of an atom is called Atomic number.
Answer:
False.
Correct statement: The sum of the number of protons and neutrons of an atom is called mass number.
Question 17.
The stable isotope of carbon (C – 12) with atomic mass 12 is used as the standard for measuring the relative atomic mass of an element.
Answer:
True.
Question 18.
The gram atomic mass of oxygen is 16 g.
Answer:
True.
Question 19.
Silicon is the most abundant element in the Earth’s crust.
Answer:
False.
Correct statement: Oxygen is the most abundant element in the Earth’s crust.
Question 20.
Except for noble gases, atoms of most of the elements are found in the combined form.
Answer:
True.
Question 21.
The number of atoms present in the molecule is called the Avogadro number.
Answer:
False.
Correct statement: The number of atoms present in the molecule is called its Atomicity.
Question 22.
O2, N2, H2, Cl2, Br2, F2, I2 are hetero diatomic molecules.
Answer:
False.
Correct statement: O2, N2, H2, Cl2, Br2, F2, I2 are homo diatomic molecules.
Question 23.
Water is an example of Hetero triatomic molecule.
Answer:
True.
Question 24.
One molecule of an element contains 6.023 × 1023 atoms and it is equal to its gram atomic mass.
Answer:
True.
Question 25.
An equal volume of all gases under similar conditions of temperature and pressure contain a different number of molecules.
Answer:
False.
Correct statement: Equal volume of all gases under similar conditions of temperature and pressure contain the same number of molecules.
Question 26.
The mathematical representation of Avogadro’slawisV/n=Constant(or)Vccn(or) V = Constant × n.
Answer:
True.
Question 27.
The molecular formula of gases can be derived using Avogadro’s law.
Answer:
True.
Question 28.
The number of moles of a sample that contains 12.046 x 1023 atoms of iron is 2.
Answer:
True.
Question 29.
The volume occupied by 14 g of Nitrogen gas is 22.4 litres.
Answer:
False.
Correct statement: The volume occupied by 14 g of Nitrogen gas is 11.2 litres.
Question 30.
Avogadro’s law determines the relation between molecular mass and absolute density.
Answer:
False.
Correct statement: Avogadro’s law determines the relation between molecular mass and vapour density.
V. Assertion and Reason
Question 1.
Assertion (A): C12H22O11 is not a simple ratio.
Reason (R): The ratio of atoms in a molecule may be fixed and integral but may not be simple.
(a) Both (A) and (R) are correct
(b) Both (A) and (R) are wrong
(c) (A) is correct but (R) is wrong
(d) (A) is wrong but (R) is correct.
Answer:
(a) Both (A) and (R) are correct
Question 2.
Assertion (A): \(_{6} \mathrm{C}^{13}\) and \(_{7} \mathrm{N}^{4}\) are called Isotones.
Reason (R): Isotones are the atoms of the different elements with different atomic number but the same mass number.
(a) Both (A) and (R) are correct
(b)Both (A) and (R) are wrong
(c) (A) is correct but (R) is wrong
(d) (A) is wrong but (R) is correct.
Answer:
(c) (A) is correct but (R) is wrong
![]()
Question 3.
Assertion (A): Nitrogen, oxygen and hydrogen are diatomic molecules.
Reason (R): N2, O2, H2 contain two atoms in one molecule and so they are a diatomic molecule.
(a) Both (A) and (R) are correct
(b) Both (A) and (R) are wrong
(c) (A) is correct but (R) is wrong
(d) (A) is wrong but (R) is correct.
Answer:
(a) Both (A) and (R) are correct
Question 4.
Assertion (A): Atoms and molecules are the building blocks of matter.
Reason (R): Atom is the ultimate particle of an element which may or may not have an independent existence.
(a) Both (A) and (R) are wrong]
(b) (A) is correct but (R) does not explain (A)
(c) Both (A) and (R) are correct
(d) (A) is wrong but (R) is correct.
Answer:
(c) Both (A) and (R) are correct
Question 5.
Assertion (A): Hydrogen, Oxygen and Ozone are called homoatomic molecules.
Reason (R): Homoatomic molecules are made up of atoms of the same element.
(a) Both (A) and (R) are wrong
(b) (A) is correct but (R) is wrong
(c) (A) is wrong but (R) is correct
(d) Both (A) and (R) are correct.
Answer:
(d) Both (A) and (R) are correct.
Question 6.
Assertion (A): Water, Ammonia (H2O, NH3) are heteroatomic molecules.
Reason (R): Most of the elementary gases and compounds consist of atoms of the same element.
(a) Both (A) and (R) are correct
(b) Both (A) and (R) are wrong
(c) (A) is correct but (R) is wrong
(d) (A) is wrong but (R) is correct.
Answer:
(c) (A) is correct but (R) is wrong
Question 7.
Assertion (A): 18 g water contains Avogadro number (6.023 × 1023) of particles.
Reason (R): 18 g of water is the molecular mass (or) 1 mole of water. One mole is defined as the amount of the substance which contains 6.023 × 1023 number of particles.
(a) (A) is correct and (R) explains (A)
(b) (A) is correct but (R) is wrong
(c) (A) is wrong but (R) is correct
(d) Both (A) and (R) are wrong
Answer:
(a) (A) is correct and (R) explains (A)
Question 8.
Assertion (A): Atoms of the same element may not be similar in all respects.
Reason (R): Atoms of the same element have the same atomic number but a different number of neutrons.
(a) Both (A) and (R) are correct
(b) (A) is correct but (R) is wrong
(c) Both (A) and (R) are wrong
(d) (A) is wrong but (R) is correct.
Answer:
(b) (A) is correct but (R)is wrong
Question 9.
Assertion (A): The atomicity of ozone is three.
Reason (R): 1 molecule of ozone contains 3 atoms of oxygen.
(a) Both (A) and (R) are correct
(b) Both (A) and(R) are wrong
(c) (A) is correct but (R) is wrong
(d) (A) is wrong but (R) is correct.
Answer:
(a) Both (A) and (R) are correct
Question 10.
Assertion (A): \(_{1} \mathrm{H}^{1}, \quad_{1} \mathrm{H}^{2},_{1} \mathrm{H}^{3}\) are the isotopes of hydrogen.
Reason (R): The atoms of the same element with the same mass number but different at numbers are called isotopes.
(a) Both (A) and (R) are correct
(b) Both (A) and (R) are wrong
(c) (A) is correct but (R) is wrong
(d) (A) is wrong but (R) is correct.
Answer:
(c) (A) is correct but (R) is wrong
Assertion (A) & Reason (R):
(i) (A) and (R) are correct. (R) explain (A)
(ii) (A) is correct (R) is wrong
(iii) (A) is wrong (R) is correct
(iv) (A) and (R) are correct. (R) does not explain (A).
Question 11.
Assertion (A): An atom is no longer indivisible.
Reason (R): The subatomic particles protons, electrons and neutrons were discovered.
Answer:
(i) (A) and (R) are correct. (R) explain (A)
Question 12.
Assertion (A): \(_{18} \mathrm{Ar}^{40}\) and \(_{20} \mathrm{Ca}^{40}\) are isobars.
Reason (R): They have the same atomic mass but a different atomic number.
Answer:
(i) A) and (R) are correct; (R) explain (A)
Question 13.
Assertion (A): \(_{17} \mathrm{Cl}^{35}\) and \(_{17} \mathrm{Cl}^{37}\) are isotones.
Reason (R): Atoms of the same element have the same atomic number but a different mass number.
Answer:
(iii) (A) is wrong (R) is correct
Question 14.
Assertion (A): NH3, H2O, HCl are heteroatomic molecules.
Reason (R): The molecule that consists of atoms of different elements is called heteroatomic molecules.
Answer:
(i) (A) and (R) are correct. (R) explain (A)
Question 15.
Assertion (A): \(_{6} \mathrm{C}^{13}\) and \(_{7} \mathrm{N}^{14}\) are called isotones.
Reason (R): Atoms of different elements having the same number of neutrons, but a different atomic number and different mass number are called isotones.
Answer:
(i) (A) and (R) are correct. (R) explain (A)
VI. Short Answer Questions.
Question 1.
What are isotopes? Give an example.
Answer:
Atoms of the same element that have same atomic number but different mass number are called isotopes.
e.g., \(_{1} \mathrm{H}^{1},_{1} \mathrm{H}^{2},_{1} \mathrm{H}^{3}\).
Question 2.
State Avogadro Hypothesis.
Answer:
Equal volumes of all gases under similar conditions of temperature and pressure contain equal number of molecules.
![]()
Question 3.
What are isotones? Give an example.
Answer:
Atoms of different elements having the same number of neutrons but a different atomic number and different mass numbers are called isotones.
e.g., \(_{6} \mathrm{C}^{13},_{7} \mathrm{N}^{14}\).
Question 4.
Define Mole.
Answer:
Mole is defined as the amount of substance that contains as many specified elementary particles as the number of atoms in 12 g of C-12 isotope.
It is also defined as the amount of substance which contains Avogadro number (6.023 × 1023) of particles.
Question 5.
Define
- Atomic number
- Mass number
Answer:
- The atomic number of an element is the number of protons or number of neutrons and electrons present in it.
- The mass number is the sum of the number of protons and neutrons in an atom.
Question 6.
How many grams are there in
(i) 5 moles of H2O
Answer:
5 moles of H2O = 5 × 18 = 90 g
(ii) 1 mole of Glucose (C6H12O6)
Answer:
1 mole of Glucose (C6H12O6) = 180 g
Question 7.
Define molecule.
Answer:
A molecule is a combination of two or more atoms held together by the strong chemical force of attraction, i.e. Chemical bonds.
Question 8.
What is homo atomic molecule? Give two examples.
Answer:
If the molecule is made of similar kind of atoms, then it is called homoatomic molecule. e.g. H2, Cl2
Question 9.
What is a heteroatomic molecule? Give two examples.
Answer:
The molecule that consists of atoms of different elements is called a heteroatomic molecule. e.g. HCl, H2O
Question 10.
Consider the following and classify them on the basis of their atomicity.
H2, CCl4, O3, BF3, HCl, HNO3, C12H22O11, NO, Cl2, He, Au, P4
- Monoatomic molecule – He, Au
- Homo diatomic molecule – H2, Cl2
- Homo triatomic molecule – O3
- Homo polyatomic molecule – P4
- Hetero diatomic molecule – HCl, NO
- Hetero polyatomic molecule – CCl4, BF3, HNO3, C12H22O11.
Question 11.
Define Relative molecular mass.
Answer:
The Relative molecular mass of a molecule is the ratio between the mass of one molecule of the substance to 1 / 12th mass of an atom of Carbon – 12 isotope.
Question 12.
Define Mole.
Answer:
The mole is the amount of the substance that contains as many elementary entities as there are atoms in exactly 12 g of the carbon – 12 isotope.
Question 13.
Define the Avogadro number.
Answer:
The actual number of atoms in 12 g of carbon – 12 is called the Avogadro number.
It is equal to 6.023 × 1023 (NA).
Question 14.
What is meant by percentage composition? What is its use?
Answer:
The percentage composition of a compound represents the mass of each element present in 100 g of the compound. It is useful to determine the empirical formula and molecular formula.
Question 15.
State Avogadro hypothesis (or) Avogadro’s Law.
Answer:
The Avogadro’s law states that “equal volume of all gases under similar conditions of temperature and pressure contain the equal number of molecules”.
[V ∝ n].
Question 16.
What are the applications of Avogadro’s Law?
Answer:
- It explains Gay – Lussac’s law.
- It helps in the determination of atomicity of gases.
- The molecular formula of gases can be derived using Avogadro’s law.
- It determines the relation between molecular mass and vapour density.
- It helps to determine the gram molar volume of all gases, (i.e, 22.4 litres at S.T.P).
Question 17.
How is Average atomic mass calculated?
Answer:
The average atomic mass of an element is calculated by adding the masses of its isotopes, each multiplied by their natural abundance on the Earth.
Question 18.
Define Vapour density.
Answer:
The vapour density is defined as the ratio between the masses of equal volumes of a gas (or vapour) and hydrogen under the same condition.
![]()
Question 19.
Write the relationship between
- Atomicity and Molecular mass
- Molecular mass and Vapour density.
Answer:
- Atomicity = \(\frac{\text { Molecular mass }}{\text { Atomic mass }}\)
- Molecular mass = 2 × Vapour density
Question 20.
Distinguish between isotopes and isobars.
Answer:
| Isotopes | Isobars |
| The atoms of the same element with same atomic number (Z) but different mass number (A) are called isotopes. e.g. \(_{17} \mathrm{Cl}^{35},_{17} \mathrm{Cl}^{37}\) |
The atoms of the different element with the same mass number (A) but different atomic number (Z) are called isobars. e.g. \(_{18} \mathrm{Ar}^{40},_{20} \mathrm{Ca}^{40}\) |
Question 21.
What are the types of molecules? Give an example for each type?
Answer:
Molecules are of two types:
- Homoatomic molecule: The molecules which are made up of atoms of the same element are called Homoatomic molecule, e.g., N2, O2, H2
- Heteroatomic molecule: The molecules which are made up of atoms of different elements are called Heteroatomic molecule, e.g., NH3, H2O, CH4
VII. HOT Questions.
Question 1.
Calculate the mass of CO2 which contains the same number of molecules as are contained in 40 g of SO2.
Answer:
Gram molecular mass of SO2 = 32 + 2(16)
= 64 g
No. of moles of SO2 = \(\frac{GivenMass}{Mol.Mass}\)
= \(\frac{40}{64}\) = 0.625 moles
∵ Equal moles contains equal number of molecules.
Mass of CO2 which contains the same number of molecules,
= 0.625 × mol. mass of CO2
= 0.625 × 44
= 27.5 g
Question 2.
A flask P contains 0.5 moles of oxygen gas. Another flask Q contains 0.4 moles of ozone gas. Which of the two flasks contains greater number of oxygen atoms?
Answer:
1 molecule of oxygen (O2) = 2 atoms of oxygen
1 molecule of ozone (O3) = 3 atoms of oxygen
In flask P:
1 mole of oxygen gas = 6.022 × 1023 molecules
0.5 mole of oxygen gas = 6.022 × 1023 × 0.5 molecules
= 6.022 × 1023 × 0.5 × 2 atoms
= 6.022 × 1023 atoms.
In flask Q:
1 mole of ozone gas = 6.022 × 1023 molecules
0.4 mole of ozone gas = 6.022 × 1023 × 0.4 molecules
= 6.022 × 1023 × 0.4 × 3 atoms
= 7.23 × 1022 atoms
Flask Q has a greater number of oxygen atoms as compared to the flask P.
Question 3.
Chlorophyll, the green pigment of plants responsible for photosynthesis contain 2.68% of Mg by weight. Calculate the number of magnesium atoms in 20 g of chlorophyll.
Answer:
The weight % of Mg as 2.68
i.e.,100 g of chlorophyll contains 2.68 g of Mg
∴ 2 g of chlorophyll will contain Mg
\(\frac{2.68}{100}\) × 20
= 0.5369
1 mole of Mg = 24 g = 6.023 × 1023 atoms
∴ 0.0536 g of Mg will have = \(\frac{6.023×10^{23}}{24}\) × 0.536
= 0.1345 × 1023 atoms of Mg = 1.345 × 1022
Number of Magnesium atoms present in 20 g of chlorophyll is 1.345 × 1022
Question 4.
In three moles of ethane (C2H6), calculate the following:
- Number of moles of carbon atoms
- Number of moles of hydrogen atoms
- Number of molecules of ethane
Answer:
- 1 mole of C2H6 contains 2 moles of carbon atoms
3 moles of C2H6 will C – atoms = 6 moles - 1 mole of C2H6 contains 6 moles of hydrogen atoms
3 moles of C2H6 will contain H-atoms = 18 moles - 1 mole of C2H6 contains Avogadro’s number. i.e., 6.023 × 1023 molecules.
3 moles of C2H6 will contain ethane molecules = 3 × 6.023 × 1023 = 18.06 × 1023 molecules.
Question 5.
If ten volumes of dihydrogen gas react with five volumes of dioxygen gas, how many volumes of water vapour could be produced?
Answer:
H2 and O2 react according to the equation
H2 (g) + O2 (g) → 2H2O (g)
Thus, 2 volumes of H2 react with 1 volume of O2 to produce 2 volumes of water vapour.
Hence, 10 volumes of H2 will react completely with 5 volumes of O2 to produce 10 volumes of water vapour.
VIII. Long Answer Questions.
Question 1.
What are the differences between atoms and molecules?
Answer:
| Atom | Molecule |
| An atom is the smallest particle of an element | A molecule is the smallest particle of an element or compound. |
| Atom does not exist in the free state except in a noble gas | The molecule exists in the free state |
| Except some of the noble gas, other atoms are highly reactive | Molecules are less reactive |
| Atom does not have a chemical bond | Atoms in a molecule are held by chemical bonds |
| Example: Na | Example: N2 |
Question 2.
Write the applications of Avogadro’s Law.
Answer:
(i) It explains Gay-Lussac’s law.
(ii) It helps in the determination of atomicity of gases.
(iii) Molecular formula of the gases can be derived.
(iv) It determines the relation between molecular mass and vapour density.
(v) It helps to determine gram molar volume of all gases
![]()
Question 3.
State and explain the applications of Avogadro’s law.
(OR)
Give any two applications of Avogadro’s law.
(OR)
Write any three applications of Avogadro’s law.
Answer:
Avogadro’s law: Equal volumes of all gases under the same conditions of temperature and pressure contain an equal number of molecules.
Applications of Avogadro’s law:
- It is used to determine the atomicity of gases.
- It is helpful in determining the molecular formula of gaseous compounds.
- It establishes the relationship between the vapour density and molecular mass of a gas.
- It gives the value of the molar volume of gases at STP. Molar volume of a gas at STP = 22.4 litres.
- It explains Gaylussac’s law effectively.
Question 4.
Explain the classification of molecules based on atomicity.
Answer:
In accordance with the number of atoms present in the molecules, they are classified as monoatomic, diatomic, triatomic and polyatomic molecules showing that they contain one, two, three or more than 3 atoms respectively.
| Atomicity | Number of atoms per molecule | Example |
| Monoatomic molecule | 1 | Helium (He), Neon (Ne) metals (Fe, Cu) |
| Diatomic molecule | 2 | Hydrogen (H2), Chlorine (Cl2) |
| Triatomic molecule | 3 | Ozone (O3) |
| Polyatomic molecule | >3 | Phosphorous (P4), Sulphur (S8) |
Question 5.
A compound made up of two elements A and B has A = 70%, B = 30%. Their relative number of moles in the compound are 1.25 and 1.88. Calculate.
(a) Atomic masses of the elements A and B.
(b) The molecular formula of the compound, if its molecular mass is found to be 160.
Answer:
| Elements | Relative no. of moles | Simplest molar ratio | Simplest whole no. molar ratio |
| A | 1.25 | \(\frac{1.25}{1.25}=1\) | 2 |
| B | 1.88 | \(\frac{1.88}{1.25}=1.5\) | 3 |
(a) Atomic mass of A = \(\frac{70}{1.25}\) = 56
Atomic mass of B = \(\frac{30}{1.88}\) = 16
(b) The molecular mass of the compound = 160
The molecular formula of the compound = Fe2O3
IX. Solve the following problems.
Question 1.
Calculate the gram molar mass of the following.
(a) NaOH
(b) C12H22O11
(c) H3PO4
(Atomic mass of Na – 23, O -16, H – 1, C – 12, P – 31)
Answer:
(a) NaOH (Sodium hydroxide)
GMM = 23 + 16 + 1
= 40 g
Gram molar mass of NaOH = 40 g
(b) C12H22O11 (Sucrose)
GMM = 12 × 12 + 22 × 1 + 11(16)
= 342 g
Gram molar mass of sucrose = 342 g
(c) H3 PO4 (Phosphoric acid)
GMM = 3(1) + 1(31)+ 4(16) = 98 g
Gram molar mass of Phosphoric acid = 98 g.
Question 2.
Calculate the percentage composition of oxygen and hydrogen by taking the example of H2O
Solution:
Mass % of an element = \(\frac{\text { Mass of that element in the compound }}{\text { Molar mass of the compound }} \times 100\)
Now, Molar mass of H2O = 2(1) + 16 = 18 g
Mass % of Hydrogen = \(\frac{2}{18} \times 100\) = 11.11 %
Mass % of Oxygen = \(\frac{16}{18} \times 100\) = 88.89 %.
Question 3.
What is the mass of 1 atom of Gold? (At. mass of Au = 197)
Answer:
The mass of 6.023 × 1023 atoms of Gold = 197 g
∴ The mass of 1 atom of gold = \(\frac{197}{6.023×10^{23}}\) × 1
= 3.27 × 10-22 g
Question 4.
Find the gram molecular mass of the following from the data given:
(i) H2O
(ii) CO2
(iii) NaOH
(iv) NO2
(v) H2SO4
| Element | Symbol | Atomic No. | Atomic Mass |
| Hydrogen | H | 1 | 1 |
| Carbon | C | 6 | 12 |
| Oxygen | O | 8 | 16 |
| Nitrogen | N | 7 | 14 |
| Sodium | Na | 11 | 23 |
| Sulphur | S | 16 | 32 |
Solution:
(i) H2O
Atomic mass of 2(H) = 2 × 1 = 2
Atomic mass of 1(O) = 1 × 16 = 16
Molecular mass of H2O = 2 + 16 = 18
(ii) CO2
Atomic mass of 1(C) = 1 × 12 = 12
Atomic mass of 2(O) = 2 × 16 = 32
Molecular mass of CO2 = 12 + 32 = 44 g
(iii) NaOH
Atomic mass of 1(Na) = 1 × 23 = 23
Atomic mass of 1(O) = 1 × 16 = 16
Atomic mass of 1(H) 1 × 1 = 1
Molecular mass of NaOH = 23 + 16 + 1 = 40 g
(iv) NO2
Atomic mass of 1(N) = 1 × 14 = 14
Atomic mass of 2(O) = 2 × 16 = 32
Molecular mass of NO2 = 14 + 32 = 46 g.
(v) H2SO24
Atomic mass of 2(H) = 2 × 1= 2
Atomic mass of 1(S) = 1 × 32 = 32
Atomic mass of 4(O) = 4 × 16 = 64
Molecular mass of H2SO4 = 64 + 32 + 2 = 98 g.
Question 5.
Complete the table given below.
| Element | Atomic Mass | Molecular Mass | Atomicity |
| Chlorine | 35.5 | 71 | – |
| Ozone | – | 45 | 3 |
| Sulphur | 32 | – | 8 |
Solution:
| Element | Atomic Mass | Molecular Mass | Atomicity |
| Chlorine | 35.5 | 71 | 2 |
| Ozone | 16 | 48 | 3 |
| Sulphur | 32 | 256 | 8 |
Question 6.
Fill in the blanks using the given data:
The formula of Calcium oxide is CaO. The atomic mass of Ca is 40, Oxygen is 16 and Carbon is 12.
- 1 mole of Ca (….. g) and 1 mole of the Oxygen atom (…… g) combine to form mole of CaO (….. g).
- 1 mole of Ca (…… g) and 1 mole of C (…… g) and 3 moles of the Oxygen atom (…… g) combine to form 1 mole of CaCO3 (…… g).
Solution:
- 1 mole of Ca (40 g) and 1 mole of the Oxygen atom (16 g) combine to form 1 mole of CaO (56 g).
- 1 mole of Ca (40 g) and 1 mole of C (12 g) and 3 moles of the Oxygen atom (48 g) combine to form 1 mole of CaCO3 (100 g).
Question 7.
Calculate the average atomic mass of naturally occurring magnesium using the following data.
Mg – 24 = 78.99% , Mg – 25 = 10%, Mg – 26 = 11.01%
Answer:
Average atomic mass of Magnesium = atomic mass of Mg – 24 × % + atomic mass of Mg – 25 × % + atomic mass of Mg – 26 × %
= 24 × \(\frac{78.99}{100}\) + 25 × \(\frac{10}{100}\) + 26 × \(\frac{11.01}{100}\)
= 18.9576 + 2.5 + 2.8626
= = 24.3202 amu
∴ Average atomic mass of Magnesium is 24.3202 amu
Question 8.
Analyse the table and fill in the blanks.
| Gas | Atomic mass | Molecular mass | Atomicity |
| Ozone | 16 | 48 | – |
| Nitrogen | 14 | – | 2 |
Solution:
| Gas | Atomic mass | Molecular mass | Atomicity |
| Ozone | 16 | 48 | 3 |
| Nitrogen | 14 | 28 | 2 |
Question 9.
Analyse the table and fill in the blanks.
| Substance | Mass | No.of moles |
| (a) Al | 81 g | – |
| (b) Fe | – | 0.5 |
Solution:
| Substance | Mass | No.of moles |
| (a) Al | 81 g | 3 |
| (b) Fe | 27.95 g | 0.5 |
Question 10.
When ammonia reacts with hydrogen chloride gas, it produces white fumes of ammonium chloride. The volume occupied by NH3 in glass bulb A is three times more than the volume occupied by HCl in glass bulb B at STP.
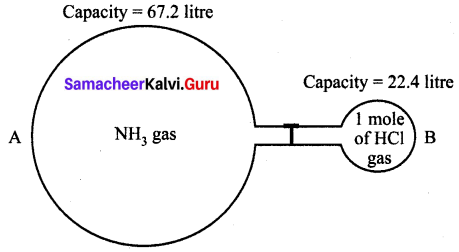
(i) How many moles of ammonia are present in glass bulb A?
(ii) How many grams of NH4Cl will be formed when the stopper is opened? (Atomic mass of N = 14, H = 1, Cl = 35.5)
(iii) Which gas will remain after completion of the reaction?
(iv) Write the chemical reaction involved in this process.
Solution:
(i) Capacity of NH3 bulb = 67.2 litre
22.4 litre of NH3 = 1 mole
67.2 litre of NH3 = \(\frac{1}{22.4} \times 67.2\) = 3 moles of NH3
(ii) Atomic mass of 1(N) = 1 × 14 = 14 g
Atomic mass of 4(H) = 4 × 1 = 4 g
Atomic mass of 1(Cl) = 1 × 35.5 = 35.5 g
Mass of NH4Cl = 53.5 g.
(iii) NH3 (Ammonia) gas will remain after the completion of the reaction.
(iv) Chemical equation of the reaction
NH3 (Ammonia) + HCl (Hydrochloric acid) → NH4Cl (Ammonium chloride)
Question 11.
Nitroglycerine is used as an explosive. The equation for the explosive reaction is
4C3H5((NO3))3 (l) → 12CO2 (g) + 10H2O (l) + 6N2 (g) + O2 (g)
(Atomic mass of C = 12, H = 1, N = 14, O = 16)
(i) How many moles does the equation show for:
(a) Nitroglycerine
(b) gas molecules produced?
(ii) How many moles of gas molecules are obtained from 1 mole of nitroglycerine?
(iii) What is the mass of 1 mole of nitroglycerine?
Solution:
(i) 4 moles of Nitroglycerine
(ii) 4 moles of Nitroglycerine produce 19 moles of gas molecules
1 mole of Nitroglycerine produces 19 / 4 = 4.75 moles
(iii) Mass of 1 mole of Nitroglycerine C3H5(NO3)3
Atomic mass of C = 12
Atomic mass of 3(C) = 3 × 12 = 36
Atomic mass of 5(H) = 5 × 1 = 5
Atomic mass of 3(N) = 3 × 14 = 42
Atomic mass of 9(O) = 9 × 16 = 144
Mass of 1 mole of Nitroglycerine = 227 g
Question 12.
Sodium bicarbonate breaks down on heating:
2NaHCO3 → Na2CO3 + H2O + CO2
(Atomic mass of Na = 23, C = 12, H = 1, O = 16)
(i) How many moles of sodium bicarbonate are there in this equation?
(ii) What is the mass of sodium bicarbonate used in this equation?
(iii) How many moles of carbon dioxide are there in this equation?
Solution:
\(2 \mathrm{NaHCO}_{3} \stackrel{\Delta}{\longrightarrow} \mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{H}_{2} \mathrm{O}+\mathrm{CO}_{2} \uparrow\)
(i) 2 moles of NaHCO3 (sodium bicarbonate) are there in the above equation.
(ii) Mass of sodium bicarbonate in this equation is mass of 2 moles of NaHCO3.
Atomic mass of 1(Na) = 1 × 23 = 23 g
Atomic mass of 1(H) = 1 × 1 = 1 g
Atomic mass of 1(C) = 1 × 12 = 12 g
Atomic mass of 3(O) = 3 × 16 = 48 g
Mass of 1 mole of NaHCO3 = 84 g
Mass of 2 moles of NaHCO3 = 84 × 2 = 168 g.
(iii) Number of moles of CO2 in this equation = 1 mole.
Question 13.
40 g of calcium was extracted from 56 g of calcium oxide (Atomic mass of Ca = 40, O = 16)
(i) What mass of oxygen is there in 56 g of calcium oxide?
(ii) How many moles of oxygen atoms are there in this?
(iii) How many moles of calcium atoms are there in 40 g of calcium?
(iv) What mass of calcium will be obtained from 1000 g of calcium oxide?
Solution:
(i) Mass of CaO = 56 g
Mass of Ca = 40 g
Mass of oxygen = 56 – 40 = 16 g
(ii) \(\frac{\text { No of moles of oxygen atom }}{\text { Mole }=\text { mass/atomic mass }}=\frac{16}{16}=1 \text { mole }\)
1 mole of oxygen atom.
(iii) \(\frac{\text { No of moles of calcium }}{\text { Mole }=\text { mass/atomic mass }}=\frac{40}{40}=1 \text { mole }\)
1 mole of calcium atom.
(iv) 56 g calcium oxide gives 40 g of calcium
1000 g of calcium oxide give = \(\frac{40}{56} \times 1000\)
= 714.285 g of calcium
= 714.29 g of calcium.
Question 14.
How many grams are there in the following?
(i) 1 mole of chlorine molecule, Cl2
(ii) 2 moles of sulphur molecules, S8
(iii) 4 moles of ozone molecules, O3
(iv) 2 moles of nitrogen molecules, N2
Solution:
(i) 1 mole of chlorine molecule Cl2
Atomic mass of chlorine = 35.5 g
Mass of 1 mole of chlorine = Atomic mass × Atomicity = 35.5 × 2 = 71 g.
(ii) 2 moles of sulphur molecules S8
Atomic mass of S8 = 8 × 32 = 256 g
Mass of 2 moles of S8 = Atomic mass × Number of moles = 256 × 2 = 512 g.
(iii) 4 moles of ozone molecule O3
Atomic mass of O3 = 3 × 16 = 48 g
Mass of 4 moles of ozone = 48 × 4 = 192 g.
(iv) 2 moles of Nitrogen molecule N2
Atomic mass of N2 = 2 × 14 = 28 g
Mass of 2 moles of Nitrogen = 28 × 2 = 56 g.
Question 15.
Find how many moles of atoms are there in:
(i) 2 g of nitrogen
(ii) 23 g of sodium
(iii) 40 g of calcium
(iv) 1.4 g of lithium
(v) 32 g of sulphur.
Solution:
(i) 2 g of nitrogen
Number of moles = \(\frac{\text { Mass }}{\text { atomic mass }}\)
Atomic mass of Nitrogen =14; Mass of Nitrogen = 2 g
Number of moles = \(\frac{2}{14}\) = 0.142 moles of Nitrogen.
(ii) 23 g of sodium
Atomic mass of sodium = 23
Mass of sodium = 23 g
Number of moles = \(\frac{\text { Mass }}{\text { atomic mass }}=\frac{23}{23}\) = 1 mole of sodium.
(iii) 40 g of calcium
Atomic mass of calcium = 40
Mass of calcium = 40 g
Number of moles = \(\frac{\text { Mass }}{\text { atomic mass }}=\frac{40}{40}\) = 1 mole of calcium.
(iv) 1.4 g of lithium
Atomic mass of lithium = 7
Mass of lithium = 1.4 g
Number of moles = \(\frac{\text { Mass }}{\text { atomic mass }}=\frac{1.4}{7}\) = 0.2 mole of lithium.
(v) 32g of sulphur
Atomic mass of sulphur = 32
Mass of sulphur = 32 g
Number of moles = \(\frac{\text { Mass }}{\text { atomic mass }}=\frac{32}{32}\) = 1 mole of sulphur.
![]()
Question 16.
Find the atomicity of chlorine, if its atomic mass is 35.5 and its molecular mass is 71.
Solution:
Atomicity = \(\frac{\text { Molecular mass }}{\text { Atomic mass }}\)
Atomicity of chlorine = \(\frac{71}{35.5}\) = 2.
Question 17.
Find the atomicity of ozone if its atomic mass is 16 and its molecular mass is 48.
Solution:
Atomicity = \(\frac{\text { Molecular mass }}{\text { Atomic mass }}\)
Atomicity of chlorine = \(\frac{48}{16}\) = 3.
Question 18.
How many atoms are present in 5 moles of oxygen?
Solution:
One mole of oxygen contains 6.023 × 1023 atoms
5 moles of oxygen contain = 5 × 6.023 × 1023
= 30.115 × 1023
= 3.0115 × 1024 atoms.
Question 19.
Calculate the number of moles in
- 81 g of Aluminium
- 4.6 g of sodium
- 5.1 g of ammonia
- 90 g of water
- 2 g of NaOH.
Solution:
No of moles = \(\frac{\text { Given mass }}{\text { Atomic mass }}\)
- No. of moles of Aluminium = \(\frac { 81 }{ 27 }\) = 3 moles of aluminium
- No. of moles of Sodium = \(\frac { 4.6 }{ 23 }\) = 0.2 moles of sodium
- No. of moles of Ammonia = \(\frac { 5.1 }{ 17 }\) = 0.3 moles of ammonia
- No. of moles of Water = \(\frac { 90 }{ 18 }\) = 5 moles of water
- No. of moles of NaOH = \(\frac { 2 }{ 40 }\) = 0.05 moles of NaOH
Question 20.
Calculate the mass of 0.5 moles of iron.
Solution:
Mass = Atomic mass × number of moles
Mass of iron = 55.9 × 0.5 = 27.95 g.
Question 21.
Find the mass of 2.5 moles of oxygen atoms.
Solution:
Mass = Atomic mass × number of moles
Mass of oxygen = 16 × 2.5 = 40 g.
Question 22.
Calculate the number of molecules in 11 g of CO2.
Solution:
Gram molecular mass of CO2 = 44 g
Number of molecules = \(\frac{\text { Avogadro number } \times \text { given mass }}{\text { Gram molecular mass }}\)
= \(\frac{6.023 \times 10^{23} \times 11}{44}\)
= 1.51 × 1023 CO2 molecules.
Question 23.
Calculate the number of molecules in 360 g of glucose.
Solution:
Number of molecules = \(\frac{\text { Avogadro number } \times \text { given mass }}{\text { Gram molar mass }}\)
Gram molar mass of glucose (C6H12O6) = (6 × 12) + (12 × 1) + (6 × 16)
= 72 + 12 + 96
= 180 g
Number of molecules = \(\frac{6.023 \times 10^{23} \times 360}{180}\)
= 6.023 × 1023 × 2
= 12.046 × 1023 molecules.
(or)
1.2046 × 1024 glucose molecules.
![]()
Question 24.
Calculate the mass of 18.069 × 1023 molecules of SO2?
Solution:
Mass of the substance = \(\frac{\text { Gram molecular mass } \times \text { Number of particles }}{\text { Avogadro number }}\)
Gram molecular mass of SO2 = 32 + 16(2) = 64 g
Mass of SO2 = \(\frac{64 \times 18.069 \times 10^{23}}{6.023 \times 10^{23}}\)
= 64 × 3 = 192 g.
Question 25.
Calculate the mass of glucose in 2 × 1024 molecules.
Solution:
Gram molecular mass of glucose = 180 g
Mass of glucose = \(\frac{180 \times 2 \times 10^{24}}{6.023 \times 10^{23}}\) = 597.7 g
Question 26.
Calculate the mass of 12.046 × 1023 molecules of CaO.
Solution:
Gram molecular mass of CaO = 40 + 16 = 56 g
Mass of CaO = \(\frac{56 \times 12.046 \times 10^{23}}{6.023 \times 10^{23}}\)
56 × 2 = 112 g.
Question 27.
Calculate the number of moles for a substance containing 3.0115 × 1023 molecules in it.
Solution:
Number of moles = \(\frac{\text { No. of molecules }}{\text { Avogadro number }}\)
= \(\frac{12.046 \times 10^{22}}{6.023 \times 10^{23}}\)
= 0.5 mole.
Question 28.
Calculate the number of moles in 12.046 × 1022 atoms of copper.
Solution:
No. of moles of atoms
\(\begin{array}{l}{=\frac{\text { No. of atoms }}{\text { Avogadro number }}} \\ {=\frac{12.046 \times 10^{22}}{6.023 \times 10^{23}}=2 \times 10^{-1}} \\ {=0.2 \text { mole. }}\end{array}\).
Question 29.
Calculate the number of moles in 24.092 × 1022 molecules of water.
Solution:
Number of moles =
\(\begin{array}{l}{=\frac{\text { No. of molecules }}{\text { Avogadro number }}} \\ {=\frac{24.092 \times 10^{22}}{6.023 \times 10^{23}}}\end{array}\)
= 4 × 1022 × 10-23
= 4 × 10-1
= 0.4 mole.
Question 30.
Which one of the following will have largest number of atoms?
- 1 g Au (s)
- 1 g Na (s)
- 1 g Li (s)
- 1 g of Cl2 (g)
(Atomic masses: Au = 197, Na = 23, Li = 7, Cl = 35.5 amu)
Solution:
- 1 g Au = \(\frac{1}{197}\) mol = \(\frac{1}{197}\) × 6.02 × 1023 atoms
- 1 g Na = \(\frac{1}{23}\) mol = \(\frac{1}{23}\) × 6.02 × 1023 atoms
- 1 g Li = \(\frac{1}{7}\) mol = \(\frac{1}{7}\) × 6.02 × 1023 atoms
- 1 g Cl2 = \(\frac{1}{71}\) mol = \(\frac{1}{71}\) × 6.02 × 1023molecules = \(\frac{2}{71}\) × 6.02 × 1023 atoms.
Thus, 1 g of Li has the largest number of atoms.
Question 31.
Calculate the number of atoms in each of the following:
(i) 52 moles of He
(ii) 52 u of He
(iii) 52 g of He
Solution:
(i) 1 mol of He = 6.022 × 1023 atoms
52 mol of He = 52 × 6.022 × 1023 atoms = 3.131 × 1025 atoms.
(ii) 1 atom of He = 4 u of He
4 u of He = 1 atom of He
52 u of He = \(\frac{1}{4}\) × 52 atoms = 13 atoms.
(iii) 1 mole of He = 4 g = 6.022 × 1023 atoms
52 g of He = \(\frac{6.022 \times 10^{23}}{4} \times 52\) atoms = 7.8286 × 1024 atoms.
![]()
Question 32.
Calculate the number of moles in each of the following.
(i) 392 g of sulphuric acid
(ii) 44.8 litres of sulphur dioxide at N.T.P.
(iii) 6.022 × 1022 molecules of oxygen
(iv) 8 g of calcium
Solution:
(i) 392 g of sulphuric acid
Molar mass of H2SO4 = 2 × 1 + 32 + 4 × 16 = 98 g
98 g of sulphuric acid = 1 mol
392 g of sulphuric acid = 1 mol × \(\frac{392 g}{(98 g)}\) = 4 mol.
(ii) 44.8 litres of sulphur dioxide at N.T.P.
22.4 litres of sulphur dioxide at N.T.P. = 1 mol
44.8 litres of sulphur dioxide at N.T.P. = \(\frac{1 \mathrm{mol}}{(22.4 \mathrm{L})} \times(44.8 \mathrm{L})\) = 2.0 mol.
(iii) 6.022 × 1022 molecules of oxygen
6.022 × 1022 molecules of oxygen = 1 mol
6.022 × 1022 molecules of oxygen = 1 mol × \(\frac{6.022 \times 10^{22}}{6.022 \times 10^{23}}\) = 0.1 mol.
(iv) 8 g of calcium
Gram atomic mass of Ca = 40 g
40 g of calcium = 1 mol
8.0 g of calcium = 1 mol × \(\frac{(8.0 \mathrm{g})}{(40 \mathrm{g})}\) = 0.2 mol.
Question 33.
The density of water at room temperature is 1.0 g/mL. How many molecules are there in a drop of water if its volume is 0.05 mL?
Solution:
Volume of a drop of water = 0.05 mL
Mass of a drop of water = Volume × density = (0.05 mL) × (1.0 g/mL) = 0.05 g
Gram molecular mass of water (H2O) = 2 × 1 + 16 = 18 g
18 g of water = 1 mol
0.05 g of water = \(\frac{1 \mathrm{mol}}{(18 \mathrm{g})} \times(0.05 \mathrm{g})\) = 0.0028 mol
No. of molecules present
1 mole of water contain molecules = 6.022 × 1023
0.0028 mole of water contain molecules = 6.022 × 1023 × 0.0028 = 1.68 × 1021 molecules.
Question 34.
Calculate the total number of electrons present in 1.6 g of methane.
Solution:
(i) Molar mass of methane (CH4) = 12 + 4 × 1 = 16 g
16 g of methane contain molecules = 6.022 × 1023
1.6 g of methane contain molecule = \(=\frac{6.022 \times 10^{23}}{(16 \mathrm{g})} \times(1.6 \mathrm{g})\) = 6.022 × 1022
(ii) Number of electrons in 6.022 × 1022 molecules of methane
1 molecule of methane contains electrons = 6 + 4 = 10
6.022 × 1022 molecules of methane contain electrons = 6.022 × 1022 × 10 = 6.022 × 1023
Question 35.
The Vapour Density of a gaseous element is 5 times that of oxygen under similar conditions. If the molecule is triatomic, what will be its atomic mass?
Solution:
Molecular mass of oxygen = 32 u
Density of oxygen = \(\frac{32}{2}\) = 16 u
Density of gaseous element = 16 × 5 = 80 u
Molecular mass of gaseous element = 80 × 2 = 160 u
Atomicity of the element = 3
Atomic mass of the element = \(\frac{\text { Molecular mass }}{\text { Atomicity }}=\frac{160}{3}\) = 53.33 u.