Students can Download Samacheer Kalvi 10th Science Model Question Paper 4 English Medium Pdf, Samacheer Kalvi 10th Science Model Question Papers helps you to revise the complete Tamilnadu State Board New Syllabus and score more marks in your examinations.
Tamil Nadu Samacheer Kalvi 10th Science Model Question Paper 4 English Medium
General Instructions:
- The question paper comprises of four parts
- You are to attempt all the questions in each part. An internal choice of questions is provided wherever applicable.
- All questions of Part I, II, III and IV are to be attempted separately.
- Question numbers 1 to 12 in Part I are Multiple Choice Questions of one mark each.
These are to be answered by writing the correct answer along with the corresponding option code. - Question numbers 13 to 22 in Part II are of two marks each. Any one question should be answered compulsorily.
- Question numbers 23 to 32 in Part III are of four marks each. Any one question should be answered compulsorily.
- Question numbers 33 to 35 in Part IV are of seven marks each. Draw diagrams wherever necessary.
Time: 3 Hours
Maximum Marks: 75
Part – I
(i) Answer all the questions. [12 × 1 = 12]
(ii) Choose the most suitable answer and write the code with the corresponding answer.
Question 1.
Impulse is equal to _________.
(a) rate of change of momentum
(b) rate of force and time
(c) change of momentum
(d) rate of change of mass
Answer:
(c) change of momentum
Question 2.
The value of universal gas constant ________.
(a) 3.81 mol-1 KJ-1
(b) 8.03 J mol-1 K-1
(c) 1.38 mol-1 KJ-1
(d) 8.31 J mol-1 K-1
Answer:
(d) 8.31 J mol-1 K-1
![]()
Question 3.
The safe limit of receiving the radiation is about _______.
(a) 1R
(b) 0.1 R
(c)100 R
(d) 10 R
Answer:
(b) 0.1 R
Question 4.
The gram molecular mass of oxygen molecule is ________.
(a) 16 g
(b) 18 g
(c) 32 g
(d) 17 g
Answer:
(c) 32 g
Question 5.
The basis of modem periodic law is _________.
(a) atomic number
(b) atomic mass
(c) Isotopic mass
(d) number of neutrons
Answer:
(a) atomic number
![]()
Question 6.
A solution is a ______ mixture.
(a) homogeneous
(b) heterogeneous
(c) homogeneous and heterogeneous
(d) non-homogeneous
Answer:
(a) homogeneous
Question 7.
Which is the sequence of correct blood flow _______.
(a) ventricle – atrium – vein – arteries
(b) atrium – ventricle – veins – arteries
(c) atrium – ventricle – arteries – vein
(d) ventricles – vein – atrium – arteries
Answer:
(c) atrium – ventricle – arteries – vein
Question 8.
Polyphagia is a condition seen in ______.
(a) Diabetes insipidus
(b) Diabetes mellitus
(c) Obesity
(d) AIDS
Answer:
(b) Diabetes mellitus
Question 9.
The xylem and phloem arranged side by side on same radius is called _______.
(a) radial
(b) amphivasal
(c) conjoint
(d) None of these
Answer:
(c) conjoint
Question 10.
Which is formed during anaerobic respiration?
(a) Carbohydrate
(b) Ethyl alcohol
(c) Acetyl CoA
(d) Pyruvate
Answer:
(b) Ethyl alcohol
![]()
Question 11.
Which is used to build scripts?
(a) Script area
(b) Block palette
(c) stage
(d) Sprite
Answer:
(a) Script area
Question 12.
Rice normally grows well in alluvial soil, but _______ is a rice variety produced by mutation breeding that grows well in saline soil.
(a) Atlas 66
(b) Triticale
(c) Protina
(d) Atomita-2-rice
Answer:
(d) Atomita-2-rice
Part – II
Answer any seven questions. (Q.No: 22 is compulsory) [7 × 2 = 14]
Question 13.
Why a spanner with a long handle is preferred to tighten screws in heavy vehicles?
Answer:
This is because turning effect to tighten the screws depends upon the perpendicular distance of the applied force from the axis of rotation in power arm. Larger the power arm less is the force required to turn the screws. So spanner is provided with a long handle.
![]()
Question 14.
State Snell’s law.
Answer:
The ratio of the sine of the angle of incidence and sine of the angle of refraction is equal to the ratio of refractive indices of the two media. This law is also known as Snell’s law.
\(\frac{\sin i}{\sin r}=\frac{\mu_{2}}{\mu_{1}}\)
Question 15.
What is co-efficient of cubical expansion?
Answer:
The ratio of increase in volume of the body per degree rise in temperature to its unit volume is called as coefficient of cubical expansion.
Question 16.
Define Relative atomic mass.
Answer:
Relative atomic mass of an element is the ratio between the average mass of its isotopes to
l/12th part of the mass of a carbon-12 atom. It is denoted as Ar.
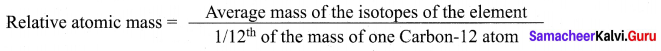
Question 17.
Differentiate aqueous and non-aqueous solution:
Answer:
Aqueous solution:
- Water acts as a solvent
- E.g. Common salt in water
Non -Aqueous solution:
- Any liquid, other than water, acts as a solvent.
- E.g. Sulphur dissolved in carbon disulphide
Question 18.
How is diastema formed in rabbit?
Answer:
Diastema is a gap between the incisors and premolar. It is formed due to the absence of canine. It helps in mastication and chewing of food in herbivorous animals.
![]()
Question 19.
What is the role of parathormone?
Answer:
The parathormone regulate calcium and phosphorus metabolism in the body. They act on bone, kidney and intestine to maintain blood calcium levels.
Question 20.
Identify the parts A, B, C and D?
Answer:
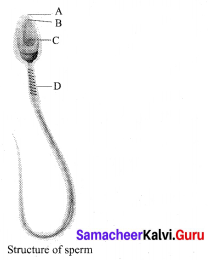
A – Head
B – Acrosome
C – Nucleus
D – Mitochondria
Question 21.
Write any two importance of Ethnobotany.
Answer:
- It provides traditional uses of plant.
- It gives information about certain unknown and known useful plants.
Question 22.
The focal length of the concave lens is 7 m. Calculate the power of lens.
Answer:
Given f = -7 m
Power of lens = \(\frac{1}{f}=\frac{1}{-7}\) =
= -0.14 dioptre or -0.14 D
Part – III
Answer any seven questions (Q.No: 32 is compulsory) [7 × 4 = 28]
Question 23.
Write the applications of universal law of gravitation.
Answer:
- Dimensions of the heavenly bodies can be measured using the gravitation law. Mass of the Earth, radius of the Earth, acceleration due to gravity, etc. can be calculated with a higher accuracy. .
- Helps in discovering new stars and planets.
- One of the irregularities in the motion of stars is called ‘Wobble’ that lead to the disturbance in the motion of a planet nearby. In this condition the mass of the star can be calculated using the law of gravitation.
- Helps to explain germination of roots is due to the property of geotropism, which is the property of a root responding to the gravity.
- Helps to predict the path of the astronomical bodies.
![]()
Question 24.
(i) Differentiate convex lens and concave lens.
Answer:
| Convex lens | Concave lens |
| The lens which is thicker at the centre than at the edges.. | The lens which is thinner at the centre than at the edges |
| A beam of light passing through it is converged to a point. | A beam of light passing through it, is diverged or spreadout. |
| It is called as converging lens. | It is called as diverging lens. |
(ii) Define dispersion of light.
Answer:
When a beam of white light or composite light is refracted through any transparent media such as glass or water, it is split into its component colours. This phenomenon is called as‘dispersion of light’.
Question 25.
(i) Three resistors of resistance 5 ohm, 3 ohm and 2 ohm are connected in series with 10 V battery. Calculate their effective resistance and the current flowing through the circuit.
Answer:
R1 = 5 Ω, R2 = 3 Ω, R3 = 2 Ω, V = 10 V
Rs = R1 + R2 + R3
= 5 + 3 + 2= 10 Ω
Hence Rs = 10 Ω
The current, I = \(\frac{V}{R_{s}}=\frac{10}{10}\) = IA
(ii) An alloy of nickel and chromium is used as the heating element. Why?
Answer:
An alloy of nickel and chromium is used as the heating element. Because:
- It has high resistivity
- It has a high melting point
- It is not easily oxidized.
Question 26.
State and explain the applications of Avogadro’s law.
Answer:
Avogadro’s law : Equal volumes of all gases under the same conditions of temperature and pressure contain an equal number of molecules.
Applications of Avogadro’s law:
- It is used to determine the atomicity of gases.
- It is helpful in determining the molecular formula of gaseous compounds.
- It establishes the relationship between the vapour density and molecular mass of a gas.
- It gives the value of molar volume of gases at STP.
Molar volume of a gas at STP = 22.4 litres. - It explains Gay lussac’s law effectively.
Question 27.
Explain the types of double displacement reactions with examples.
Answer:
There are two major classes of double displacement reactions. They are,
(i) Precipitation Reactions:
When aqueous solutions of two compounds are mixed, if they react to form an insoluble compound and a soluble compound, then it is called precipitation reaction.
pb(NO3)2(aq) + 2KI(aq) → PbI2(s) ↓+ 2KNO3(aq)
(ii) Neutralisation Reactions:
Another type of displacement reaction in which the acid reacts with the base to form a salt and water. It is called ‘neutralisation reaction’ as both acid and base neutralize each other.
NaOH(aq) + HCl1(aq) → NaCl(aq) + H2O(l)
![]()
Question 28.
(i) Explain the structure of an atom bomb.
Answer:
Principle:
- Uncontrolled chain reaction
- Release large amount of energy in short time
Structure:
- Fissile material whose mass is sub – critical.
- Cylindrical fissile material is fit into void.
- Cylinder is injected into the void, using conventional explosive
- Two pieces together to form super – critical mass, leads to explosion.
- Tremendous amount of energy is released in the form of heat, light and radiation.
- γ radiation, affects the living creatures
- This type of atom bombs were exploded at Hiroshima and Nagasaki in Japan during Second World War.
(ii) Calculate the pH of 1.0 × 10-4 molar solution of HNO3.
Answer:
[H+] = 1.0 × 10-4 m, pH = ?
pH = – log10 [H+]
= – log10 1 × 10-4
[M+] = – log10 1– log10 10-4
= 0 – (-4) log10 10 ∴ log10 10 = 1
= 4 × 1
pH =4
Question 29.
With a neat labelled diagram explain the structure of a neuron.
Answer:
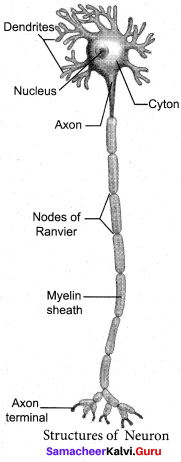
A neuron or nerve cell is the structural and functional unit of the nervous system. It consists of Cyton, Dendrites and Axon.
(a) Cyton: Cyton has a central nucleus with the abundant – cytoplasm called neuroplasm. The cytoplasm has large granular body called Nissel’s granules and the other cell organelles like mitochondria, ribosomes, lysosomes, and endoplasmic reticulum.
(b) Dendrites: These are the numerous branched cytoplasmic processes, that project from the surface of the cell body. They conduct nerve impulses, towards the cyton.
(c) Axon: The axon is a single, elongated, slender projection. The end of axon terminates as fine branches, which terminate into knob like swellings called synaptic knob. The plasma membrane of axon is called axolemma, while the cytoplasm is called axoplasm. It carries impulses away from the cyton.
The axon may be covered by a protein sheath called myelin sheath, which is further covered by a layer of Schwann cells called Neurilemma. Myelin sheath breaks at intervals, by depressions called Nodes of Ranvier. The region between the nodes is called as internode.
![]()
Question 30.
(i) What are the various routes by which transmission of human immuno deficiency virus takes place?
Answer:
AIDS is not transmitted by touch or any physical contact. HIV is generally transmitted by
- Sexual contact with infected person.
- Use of contaminated or infected blood or blood products, needles or syringes.
- By the use of contaminated needles or syringes.
- From infected mother to her child through placenta.
(ii) Name three improved characteristics of wheat that helped India to achieve high productivity.
Answer:
- High yielding
- Semi-dwarf
- Fertilizer responsive
Question 31.
(i) Kavitha gave birth to a female baby. Her family members say that she can give birth to only female babies because of her family history. Is the statement given by her family members true. Justify your answer.
Answer:
No, the statement is not true. Sex determination is a chance of probability as to which category of sperm fuses with egg.
If the egg [X] is fused by the X-bearing sperm, an XX individual (female) is produced.
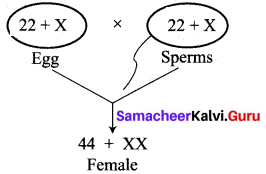
If the egg [X] is fused by the Y-bearing sperm an XY individual (male) is produced.
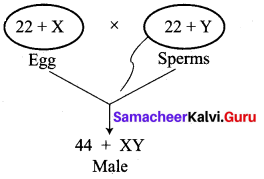
The sperm, produced by the father, determines the sex of the child. The mother is not responsible in determining the sex of the child.
(ii) Who discovered Rh factor? Why was it named so?
Answer:
The Rh factor was discovered by Landsteiner and Wiener in 1940 in Rhesus monkey. Hence the name Rh factor.
Question 32.
Describe and name three stages of cellular respiration that aerobic organisms use to obtain energy?
Answer:
Stages of cellular respiration
(i) Glycolysis: It takes place in cytoplasm of the cell. It is the break down of one molecule of Glucose (6 Carbon) into two molecules of Pyruvic acid (3 Carbon).

(ii) Kreb’s cycle: This occurs in mitochondria matrix. During this cycle, oxidation of pyruvic acid result into CO2 and water.
![]()
(iii) Electron transport chain(ETC):
ETC is located on the inner membrane of the mitochondria.
(a) NADH2 and FADH2 molecules formed during Glycolysis and Kreb’s cycle are oxidised to NAD+ and FAD+ to release the energy via electrons.
(b) The electrons, as they move through the system, release energy which is trapped by ADP to synthesize ATP. This is called Oxidative Phosphorylation. During this process, oxygen which is the acceptor of electrons get reduced to water.
Part – IV
(1) Answer all the questions. [3 × 7 = 21]
(2) Each question carries seven marks.
(3) Draw diagram wherever necessary.
Question 33.
(a) (i) State Newton’s third law of motion.
Answer:
‘For every action, there is an equal and opposite reaction. They always act on two different bodies’.
(ii) Deduce the equation of a force using Newton’s second law of motion.
Answer:
“The force acting on a body is directly proportional to the rate of change of linear momentum of the body and the change in momentum takes place in the direction of the force”.
Let, ‘m’ be the mass of a moving body, moving along a straight line with an initial speed ‘u’. After a time interval of ‘t’ the velocity of the body changes to ‘v’ due to the impact of an unbalanced external force F.
Initial momentum of the body Pi = mu
Final momentum of the body Pf = mv
Change in momentum Δp = Pf – Pi
= mv – mu
By Newton’s second law of motion,
Force, F ∝ rate of change of momentum
F ∝ change in momentum / time
F ∝ \(\frac{m v-m u}{t}\)
F = \(\frac{k m(v-u)}{t}\)
Here, k is the proportionality constant, k = 1 in all systems of units. Hence,
F = \(\frac{m(v-u)}{t}\)
Since, acceleration = change in velocity / time, a = (v – u)/t. Hence, we have
F = m × a
Force = mass × acceleration
- No external force is required to maintain the motion of a body moving with uniform velocity.
- When the net force acting on a body is not equal to zero, then definitely the velocity of the body will change.
- Thus, change in momentum takes place in the direction of the force. The change may take place either in magnitude or in direction or in both.
[OR]
(b) A piece of wire of resistance 10 ohm is drawn out so that its length is increased to three times its original length. Calculate the new resistance.
Answer:
Specific resistance (ρ) = \(\frac{\mathrm{RA}}{l}\)
R = \(\frac{\rho l}{\mathrm{A}}\)
When the length is increased by three and then the area of cross section is reduced by three
Resistance of wire R = 10 Ω
New resistance R’ = \(\frac{\rho(3 l)}{(A / 3)}\)
= ρ (3l) x \(\frac{3}{\mathrm{A}}\)
R’ = \(\frac{9 \rho l}{\mathrm{A}}\)
R’ = 9 x R = 9 x 10
R’ = 90 Ω
![]()
Question 34.
(a) (i) Calculate the mass of the 0.023 x 1020 molecules of H2O.
Answer:
Molecular mass of H2O = H × 2 + 0 × 1
= 1 × 2 + 16 × 1
= 2 + 16 = 18

= 10 × 18
Mass of an atom = 0.018 g
(ii) Calculate the moles of 12 g of magnesium.
Answer:
Number of moles = \(\frac{\text { Given mass }}{\text { molecular mass }}\)
= \(\frac{12}{24}=\frac{1}{2}\) = 0.5
∴ 12 g magnesium makes 0.5 moles.
(iii) Calculate the number of molecules present in 50 g of Fe.
Answer:

= 5.387 × 1023
∴ 50 g Fe contain 5.387 × 1023 atoms/molecules
[OR]
(b) Explain smelting process..
Answer:
Smelting: The charge consisting of roasted ore, coke and limestone in the ratio 8 : 4 : 1 is smelted in a blast furnace by introducing it through the cup and cone arrangement at the top.
There are three important regions in the furnace.
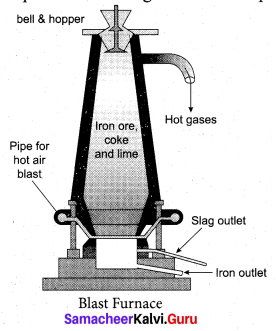
(a) The Lower Region (Combustion Zone): The temperature is at 1500°C. In this region, coke bums
with oxygen to form CO2 when the charge comes in contact with a hot blast of air.
![]()
It is an exothermic reaction since heat is liberated.
(b) The middle region (Fusion Zone): The temparature prevails at 1000° C. In this region, CO2 is reduced to CO.
![]()
Limestone decomposes to calcium oxide and CO2
![]()
These two reactions are endothermic due to absorption of heat. Calcium oxide combines with silica to form calcium silicate slag.
CaO + SiO2 → CaSiO3
(c) The Upper Region (Reduction Zone): The temperature prevails at 400°C . In this region carbon monoxide reduces ferric oxide to form a fairly pure spongy iron.
![]()
The molten iron is collected at the bottom of the furnace after removing the slag.
The iron thus formed is called pig iron. It is remelted and cast into different moulds. This iron is called cast iron.
![]()
Question 35.
(a) (i) Write any two physiological effects of ethylene.
Answer:
Physiological effects of ethylene:
- It promotes the ripening of fruits. E.g., Tomato, Apple, Mango, Banana, etc.
- It breaks the dormancy of buds,seeds and storage organs.
(ii) What is Autogamy?
Answer:
Autogamy or self pollination is the transfer of pollen grains from the anther to the stigma of same flower or another flower borne on the same plant. Eg. Hibiscus.
(iii) How will you prevent soil erosion?
Answer:
Prevention of soil erosion:
- Retain vegetation cover, so that soil is not exposed.
- Cattle grazing should be controlled.
- Crop rotation and soil management improve soil organic matter.
- Run off water should be stored in the catchment.
- Reforestation, terracing and contour ploughing.
- Wind speed can be controlled by planting trees in the form of a shelter belt.
[OR]
(b) (i) How do you differentiate Homologous organs from Analogous organs.
Answer:
| Homologous organs | Analogous organs |
| 1. Homologous organs are those inherited from common ancestors, with similar developmental pattern in embryos. | 1. The analogous organs look similar and perform similar functions but they have different origin and development pattern. |
| 2. A human hand, a front leg of cat, flipper of a whale and a bat’s, wing look dissimilar and adapted for different functions. | 2. The function of the wings of a bat, the wings of a bird and wings of an insect are similar, but their basic structures are different. |
(ii) Why are the rings of cartilage found in trachea of rabbit?
Answer:
The tracheal walls of rabbit are supported by rings of cartilage which help in the free passage of air.