Students can Download Samacheer Kalvi 10th Science Model Question Paper 3 English Medium Pdf, Samacheer Kalvi 10th Science Model Question Papers helps you to revise the complete Tamilnadu State Board New Syllabus and score more marks in your examinations.
Tamil Nadu Samacheer Kalvi 10th Science Model Question Paper 3 English Medium
General Instructions:
- The question paper comprises of four parts
- You are to attempt all the questions in each part. An internal choice of questions is provided wherever applicable.
- All questions of Part I, II, III and IV are to be attempted separately.
- Question numbers 1 to 12 in Part I are Multiple Choice Questions of one mark each.
These are to be answered by writing the correct answer along with the corresponding option code. - Question numbers 13 to 22 in Part II are of two marks each. Any one question should be answered compulsorily.
- Question numbers 23 to 32 in Part III are of four marks each. Any one question should be answered compulsorily.
- Question numbers 33 to 35 in Part IV are of seven marks each. Draw diagrams wherever necessary.
Time: 3 Hours
Maximum Marks: 75
Part – I
(i) Answer all the questions. [12 × 1 = 12]
(ii) Choose the most suitable answer and write the code with the corresponding answer.
Question 1.
One kilogram force equal to _______.
(a) 9.8 dyne
(b) 9.8 × 104N
(c) 98 × 104 dyne
(d) 980 dyne
Answer:
(c) 98 × 104 dyne
Question 2.
Temperature is the average ______ of the molecules of a substance.
(a) difference in K.E and P.E
(b) sum of P.E and K.E
(c) difference in T.E and P.E
(d) difference in K.E and T.E
Answer:
(c) difference in T.E and P.E
Question 3.
In nuclear reactor _____ is used as a control rod.
(a) barium
(b) carbon
(c) cadmium
(d) Na
Answer:
(c) cadmium
![]()
Question 4.
Mass of 1 mole of Nitrogen atom is ______.
(a)28 amu
(b) 14 amu
(c) 28 g
(d) 14 g
Answer:
(b) 14 amu
Question 5.
Which of the following law inert gases 2 electrons in the outermost shell?
(a) He
(b) Ne
(c) Ar
(d) Kr
Answer:
(a) He
Question 6.
The number of components in a binary solution is ________.
(a) 2
(b) 3
(c) 4
(d) 5
Answer:
(a) 2
![]()
Question 7.
In an healthy adult during normal resting condition the blood pressure is ______.
(a) 160 mm/80 mm Hg
(b) 140 mm/80 mm Hg
(c) 120 mm/80 mm Hg
(d) 140 mm/60 mm Hg
Answer:
(c) 120 mm/80 mm Hg
Question 8.
Coronary heart disease is due to ______.
(a) Streptococci bacteria
(b) Inflammation of pericardium
(c) Weakening of heart valves
(d) Insufficient blood supply to heart muscles
Answer:
(d) Insufficient blood supply to heart muscles
Question 9.
Glycolysis takes place in _______.
(a) Cytoplasm
(b) Mitochondria
(c) Inner mitochondrial membrane
(d) Nucleus
Answer:
(a) Cytoplasm
Question 10.
________ is the ATP factory of the cell.
(a) Mitochondria
(b) Chloroplast
(c) Ribosomes
(d) Nucleus
Answer:
(a) Mitochondria
Question 11.
The “use and disuse theory” was proposed by ________.
(a) Charles Darwin
(b) Ernst Hacc Kel
(c) Jean Baptiste Lamark
(d) Gregor Mendel
Answer:
(c) Jean Baptiste Lamark
![]()
Question 12.
Which is used to edit programs?
(a) Inkscape
(b) Script editor
(c) Stage
(d) Sprite
Answer:
(b) Script editor
Part – II
Answer any seven questions. (Q.No: 22 is compulsory) [7 × 2 = 14]
Question 13.
Define moment of a couple.
Answer:
When two equal and unlike parallel forces applied simultaneously at two distinct points constitute couple. A couple results in causes the rotation of the body. This rotating effect of a couple is known as moment of a couple.
Question 14.
What is power of accommodation of eye?
Answer:
- The ability of the eye lens to focus nearby as well as the distant objects is called power of accommodation of the eye.
- This is achieved by changing the focal length of the eye lens with the help of ciliary muscles.
Question 15.
What are the advantages of LED TV over the normal TV?
Answer:
- It has bright picture quality
- It is thinner in size
- It uses less power and consumes very less energy.
- Its life span is more
- It is more reliable
![]()
Question 16.
State Ohm’s law.
Answer:
According to Ohm’s law, at a constant temperature, the steady current ‘I’ flowing through a conductor is directly proportional to the potential difference ‘V’ between the two ends of the conductor. V = IR
Question 17.
Distinguish between the saturated and unsaturated solution.
Answer:
| Saturated solution | Unsaturated solution |
| 1. A solution in which no more solute can be dissolved in a definite amount of the solvent at a given temperature is called a saturated solution. | 1. Unsaturated solution is one that contains less solute than that of the saturated solution at a given temperature. |
| 2. e.g. 36 g of NaCl in 100 g of water at 25°C | 2. e.g. 16 g of NaCl in 100 g of water at 25°C |
Question 18.
Why is the teeth of rabbit called heterodont?
Answer:
In Rabbit, the teeth are of different types, i.e., Incisors, (I) Canines (C), Premolar (PM) and molar (M). Hence the dentition is called heterodont.
Question 19.
What is the function of Oxytocin?
Answer:
Oxytocin helps in the contraction of the smooth muscles of uterus at the time of child birth and milk ejection from the mammary gland after child birth.
Question 20.
What will happen if you cut planaria into small fragments?
Answer:
If you cut planaria into small fragments, each fragment will develop into new individual by a specialised mass of cell. It is called regeneration.
Question 21.
Match the following:

Answer:
(a) ii
(b) iii
(c) iv
(d) i
![]()
Question 22.
How much current will an electric heater coil draw from 220 V source, if the resistance of heater coil is 110Ω.
Answer:
Given,V = 220 V, R = 110 Ω; I = ?
I = \(\frac{V}{R}=\frac{220}{110}\) = 2A
Part – III
Answer any seven questions (Q.No: 32 is compulsory) [7 × 4 = 28]
Question 23.
What are the types of inertia? Give an example for each type.
Answer:
- Inertia of rest: The resistance of a body to change its state of rest is called inertia of rest. E.g: When you vigorously shake the branches of a tree, some of the leaves and fruits are detached and they fall down.
- Inertia of motion: The resistance of a body to change its state of motion is called inertia of motion. E.g: An athlete runs some distance before jumping. Because, this will help him jump longer and higher.
- Inertia of direction: The resistance of a body to change its state of direction is called inertia of direction.
E.g: When you make a sharp turn while driving a car, you tend to lean sideways.
Question 24.
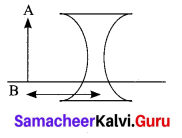
Explain the rules for obtaining images formed by a convex lens with the help of ray diagram.
Answer:
Rule-1: When a ray of light strikes the convex lens obliquely at its optical centre, it continues to follow its path without any deviation
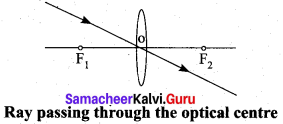
Rule-2: When a ray of light passes parallel to the principal axis of convex lens, the refracted rays is converged to the principal focus
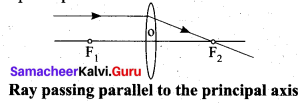
Rule-3: When a ray passing through the principal focus the refracted ray will be parallel to the principal axis
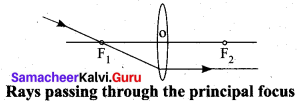
Question 25.
A piece of wire of resistance 10 ohm is drawn out so that its length is increased to three times its original length. Calculate the new resistance.
Answer:
Specific resistance (ρ) = \(\frac{\mathrm{RA}}{l}\)
R = \(\frac{\rho l}{\mathrm{A}}\)
When the length is increased by three and then the area of cross section is reduced by threeResistance of wire R = 10 Ω
New resistance R’ = \(\frac{\rho(3 l)}{(A / 3)}\)
= ρ (3l) x \(\frac{3}{\mathrm{A}}\)
R’ = \(\frac{9 \rho l}{\mathrm{A}}\)
R’ = 9 × R = 9 × 10
R’ = 90 Ω
![]()
Question 26.
Write briefly about the characteristics of hydrocarbons.
Answer:
- Lower hydrocarbons are gases at room temperature E.g. methane, ethane.
- They are colourless and odourless.
- The boiling point of hydrocarbons increases with an increase in the number of carbon atoms.
- They undergo combustion reaction with oxygen to form CO2 and water.
- Alkanes are least reactive when compared to other classes of hydrocarbons.
- Alkynes are the most reactive due to the presence of the triple bond.
- Alkanes are saturated whereas alkenes and alkynes are unsaturated.
- They are insoluble in water.
Question 27.
(i) What is Hydrated salt?
Answer:
- When ionic substances are dissolved in water to make their saturated aqueous solution, their ions attract water molecules and attached chemically in certain ratio. This process is called hydration.
- These ionic substances crystallize out from their saturated aqueous solution with a definite number of molecules of water.
- The number of water molecules found in the crystalline substance is called water of crystallization.
- Such salts are called hydrated salts
- Eg. Blue vitriol – CuSO4.5H2O
(ii) How is ethanoic acid prepared from ethanol? Give the chemical equation?
Answer:
Ethanoic acid is prepared by the oxidation of ethanol in the presence of alkaline potassium permanganate or acidified potassium dichromate.
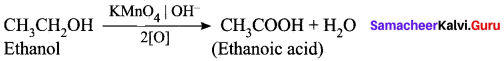
Question 28.
Differentiate voluntary and involuntary action.
Answer:
Voluntary Action:
| Voluntary Action | Involuntary Action |
| 1. Controlled by Cerebrum | 1. Controlled by the spinal cord. |
| 2. The actions are under the control of our will | 2. The actions, are not under our control |
| 3. Eg. Writing, speaking | 3. Eg. Heart beat, Breathing |
Question 29.
(a) Lable the parts of DNA in the diagram below. Explain the structure briefly.
Answer:
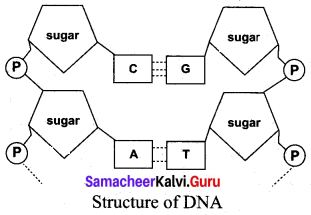
DNA is a Polynucleotide. Each nucleotide contains
(i) A sugar molecule – Deoxyribose sugar
(ii) A nitrogenous base. They are two types of nitrogeneouS bases. They are
- Purines (Adenine and Guanine)
- Pyrimidines (Cytosine and Thymine)
(iii) A phosphate group
Nucleoside = Nitrogen base + sugar
Nucleotide – Nucleoside + Phosphate
Purines and pyrimidines are linked by hydrogen bonds.
Adenine (A) links thymine (T) with two hydrogen bonds (A = T)
Cytosine (C) links Guanine (G) with three hydrogen bonds (C ≡ G)
(b) Enumerate any 4 functions of blood.
Answer:
Functions of blood:
- Transport of respiratory gases (Oxygen and CO2).
- Transport of hormones.
- It maintains proper water balance in the body.
- It acts as buffer and also helps in regulation of pH and body temperature
![]()
Question 30.
(a) What is photosynthesis? Where does it occur?
Answer:
Photosynthesis is a process by which autotrophic organism like green plants algae and chlorophyll containing bacteria utilize the energy from sunlight to synthesize their own food. Photosynthesis occur in green parts of the plant such as leaves, stems and floral buds.
(b) Write the reaction for photosynthesis?
Answer:
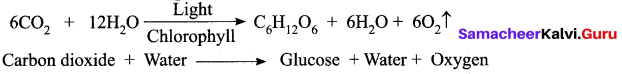
Question 31.
(a) What are the contributing factors for obesity?
Answer:
The contributing factors for obesity.
- Genetic factors
- physical inactivity
- Eating habits(Over eating)
- endocrine factors
(b) What is genetic engineering?
Answer:
Genetic engineering is the manipulation and transfer of genes from one organisms to another organisms to create a new DNA is called as recombinant DNA (rDNA). The Genetic engineering is also called as recombinant DNA technology.
Question 32.
(a) Explain linear expansion with equation.
Answer:
When a body is heated or cooled, the length of the body changes due to the change in its temperature. Then the expansion is said to be linear or longitudinal expansion. The ratio of increase in length of the body per degree rise in temperature to its unit length is called as the coefficient of linear expansion.
The value of coefficient of linear expansion is different for different materials. The equation relating the change in length and the change in temperature of a body is
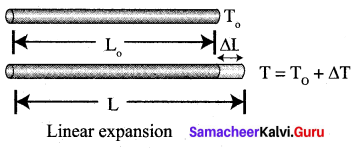
\(\frac{\Delta L}{L_{0}}\) = αLΔT
Δ L-Change in length a
L0 – Original length
ΔT – Change in temperature
αL – Coefficient of linear expansion.
(b) State Avogadro’s law.
Answer:
Equal volumes of all gases under similar conditions of temperature and pressure contain equal number of molecules.
Part – IV
(1) Answer all the questions. [3 × 7 = 21]
(2) Each question carries seven marks.
(3) Draw diagram wherever necessary.
Question 33.
(a) With the help of a circuit diagram derive the formula for the resultant resistance of three resistances connected in series.
Answer:
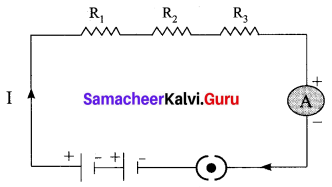
Let, three resistances R1, R2 and R3 be connected in series. Let the current flowing through them be I. According to Ohm’s Law, the potential differences V1, V2 and V3 across R1, R2 and R3 respectively, are given by:
V1 = IR1 …….. (1)
V2 = IR2 …….. (2)
V3 = IR3 ……. (3)
The sum of the potential differences across the ends of each resistor is given by
V = V1 + V2 + V3
Using equations (1), (2) and (3), we get
V = IR1 + IR2 + IR3 …… (4)
The effective resistor is a single resistor, which can replace the resistors effectively, so as to allow the same current through the electric circuit. Let, the effective resistance of the series-combination of the resistors, be Rs.
Then,
V = IRs …….. (5)
Combining equations (4) and (5) we get,
IRs = IR1 +IR2 + IR3
Rs = R1 + R2 + R3 …… (6)
Thus, When a number of resistors are connected in series, their effective resistance is equal to the sum of the individual resistances.
When ‘n’ resistors of equal resistance R are connected in series, the equivalent resistance is ‘n R’ i.e., Rs = n R
The equivalent resistance in a series combination is greater than the highest of the individual resistances.
[OR]
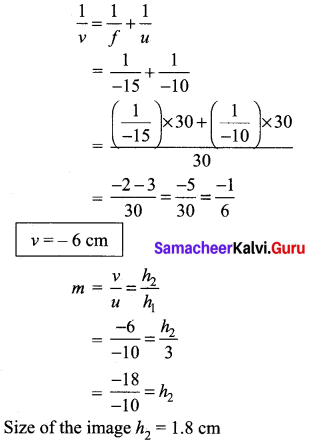
(b) (i) An object of height 3 cm is placed at 10 cm from a concave lens of focal length 15 cm. Find the size of the image.
Answer:
Given, \(\frac{1}{f}=\frac{1}{v}-\frac{1}{u}\)
f = -15 cm [left side of lens]
u =-10 cm [left side of lens]
(ii) The thunder of cloud is heard 9.8 seconds later than the flash of lightning. If the speed of sound in air is 330 ms-1. What will be the height of the cloud?
Answer:
Given, Time, t = 9.8 s
Speed of sound,v = 330 ms-1
Height of cloud, d = ?
v = d/t,
d = vt
= 330 × 9.8
d = 3234 m
![]()
Question 34.
(i) What is the mass of 1 mole of nitrogen atom?
(ii) How many moles of SO2 have same mass as 3 moles of oxygen?
(iii) How many molecules are present in 1 ml of water?
Answer:
(i) 1 × gram atomic mass of nitrogen atom
1 × 14 = 14 g
(ii) Mass of 3 moles of oxygen = 3 × 16 = 48 g
Now, mas of SO2 = 32 + [2 × 16]
= 32 + 32 = 64 g
∴ 64 g of SO2 = 1 mole
48 g SO2 = (\(\frac{1}{64}\)) × 48 = 0.75 mole
(iii) We know that density of water is 1 g /ml
Hence, 1 g water = 1 ml water
Now, We have molecular mass of water
H2O = (1 × 2) + 16 = 18 g
18 g of water contains 6.022 × 1023 molecules
1 g of water will contains = \(\frac{6.023 \times 10^{23}}{18}\) = 0.33 × 1023 molecules
So the number of molecules of water in 1 ml = 3.3 × 1022
[OR]
(b) Explain the factors influencing the rate of a reaction.
Answer:
The factors influencing the rate of a reaction are,
(i) Nature of the reactants: The reaction of sodium with hydrochloric acid is faster than that with acetic acid. Do you know why? Hydrochloric acid is a stronger acid than acetic acid and thus more reactive. So, the nature of the reactants influence the reaction rate.
2Na(s) + 2HCl(aq) → 2NaCl(aq) + H2(g) (fast)
2Na(s) + 2CH3COOH(aq) → 2CH3COONa(aq) + H2(g) (slow)
(ii) Concentration of the reactants: Changing the amount of the reactants also increases the reaction rate. The amount of the substance present in a certain volume of the solution is called ‘concentration’. More particles per volume exist in it and hence faster the reaction. Granulated zinc reacts faster with 2M hydrochloric acid than 1M hydrochloric acid.
(iii) Temperature: Most of the reactions go faster at higher temperature. Because adding heat to the reactants provides energy to break more bonds and thus speed up the reaction. Calcium carbonate reacts slowly with hydrochloric acid at room temperature. When the reaction mixture is heated the reaction rate increases.
(iv) Pressure: If the reactants are gases, increasing their pressure increases the reaction rate. This is because, on increasing the pressure the reacting particles come closer and collide frequently.
(v) Catalyst: A catalyst is a substance which increases the reaction rate without being consumed in the reaction. In certain reactions, adding a substance as catalyst speeds up the reaction. For example, on heating potassium chlorate, it decomposes into potassium chloride and oxygen gas, but at a slower rate. If manganese dioxide is added, it increases the reaction rate.
(vi) Surface area of the reactants: When solid reactants are involve in a reaction, their powdered form reacts more readily. For example, powdered calcium carbonate reacts more readily with hydrochloric acid than marble chips. Because, powdering of the reactants increases the surface area and more energy is available on collision of the reactant particles. Thus, the reaction rate is increased.
![]()
Question 35.
(a) (i) Write any 4 physiological effects of gibberellins.
(ii) Name the enzyme present in acrosome of sperm? What is its function.
(iii) What are the consequences of deforestation.
Answer:
(i) Physiological effects of gibberellins
- Gibberellins promote the production of male flowers in monoecious plants (Cucurbits).
- Gibberellins break dormancy of potato tubers.
- Gibberellins are efficient in inducing the formation of seedless fruit (Parthenocarpic fruits).
- Gibberellins stimulate extraordinary elongation of internode Eg.Corn and pea.
(ii) An enzyme, Hyaluronidase present in the acrosome help the sperm to enter the ovum during fertilization.
(iii) Deforestation is the destruction of large area of forests. It gives rise to ecological problems like floods, drought, soil erosion, loss of wild life, extinction of species, imbalance of biogeochemical cycles, alteration of climatic conditions and desertification. Therefore it is a threat to the economy, quality of life and future of the environment.
[OR]
(b) (i) List out any 3 parasitic adaptation of leech.
(ii) Explain the importance of Fossils.
Answer:
(i) Parasitic adaptation of Leech:
- Blood is sucked by pharynx
- The salivary glands produce hirudin which does not allow the blood to coagulate.
- Anterior and posterior ends of the body are provided with suckers by which the animal attaches itself to the body of the host.
(ii) Importance of Fossils:
- Fossils are useful in classification of plants.
- Fossils throw light on phytogeny and evolution of plants
- Fossil plants give a historical approach to plant kingdom
- Fossil plants can be used in the field of descriptive and comparative anatomy