Students can Download Chemistry Chapter 5 Alkali and Alkaline Earth Metals Questions and Answers, Notes Pdf, Samacheer Kalvi 11th Chemistry Solutions Guide Pdf helps you to revise the complete Tamilnadu State Board New Syllabus and score more marks in your examinations.
Tamilnadu Samacheer Kalvi 11th Chemistry Solutions Chapter 5 Alkali and Alkaline Earth Metals
Samacheer Kalvi 11th Chemistry Alkali and Alkaline Earth Metals Textual Evaluation Solved
I. Choose The Correct Answer:
Question 1.
For alkali metals, which one of the following trends are incorrect?
(a) Hydration energy : Li > Na > K > Rb
(b) Ionization energy : Li > Na > K > Rb
(c) Density : Li < Na < K < Rb
(d) Atomic size : Li < Na < K < Rb
Answer:
(c) Density : Li < Na < K < Rb
Potassium is lighter than sodium. The correct order of density is
Li < K< Na < Rb < Cs
0.54 < 0.86 < 0.97< 1.53< 1.90 (in g cm3).
Question 2.
Which of the following statements are incorrect?
(a) Li+ has minimum degree of hydration among alkali metal cations.
(b) The oxidation state of K in KO2 is +1.
(c) Sodium is used to make Na/Pb alloy.
(d) MgSO4 is readily soluble in water.
Answer:
(a) Li+ has minimum degree of hydration among alkali metal cations.
Li+ has maximum degree of hydration among alkali metal cations.
Li+ > Na+ > K+ > Rb+ > Cs+
Question 3.
Which of the following compounds will not evolve H2 gas on reaction with alkali metals?
(a) ethanoic acid
(b) ethanol
(c) phenol
(d) none of these
Answer:
(d) none of these
Hint:
All these compounds reacts with alkali metals to evolve hydrogen gas.
Question 4.
Which of the following has the highest tendency to give the reaction Aqueous –
![]()
(a) Na
(b) Li
(c) Rb
(d) K
Answer:
(b) Li.
Hint:
Hydration energy of Li+ is more and hence Li+ is stabilized in aqueous medium.
Question 5.
Sodium is stored in ………..
(a) alcohol
(b) water
(c) kerosene
(d) none of these
Answer:
(c) kerosene
![]()
Question 6.
RbO2 is ………….
(a) superoxide and paramagnetic
(b) peroxide and diamagnetic
(c) superoxide and diamagnetic
(d) peroxide and paramagnetic
Answer:
(a) superoxide and paramagnetic
Hint:
RbO2 is a super oxide which contains Rb+ and O2- ions. O2- contains one unpaired electron and hence it is paramagnetic.
Question 7.
Find the wrong statement …………
(a) sodium metal is used in organic qualitative analysis
(b) sodium carbonate is soluble in water and it is used in inorganic qualitative analysis
(c) potassium carbonate can be prepared by Solvay process
(d) potassium bicarbonate is acidic salt
Answer:
(c) Potassium carbonate can be prepared by Solvay process
Hint:
Potassium carbonate cannot be prepared by Solvay process. Potassium bicarbonate is fairly soluble in water and does not precipitate out.
Question 8.
Lithium shows diagonal relationship with
(a) sodium
(b) magnesium
(c) calcium
(d) aluminium
Answer:
(b) magnesium (diagram pending)
Question 9.
In case of alkali metal halides, the ionic character increases in the order
(a) MF < MCl < MBr < MI
(b) MI < MBr < MCl < MF
(c) MI < MBr < MF < MCl
(d) none of these
Answer:
(b) MI < MBr < MCl < MF
Hint:
Ionic character (difference in electronegativity) MI < MBr < MCl < MF
Question 10.
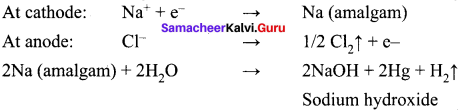
In which process, fused sodium hydroxide is electrolysed for extraction of sodium?
(a) Castner’s process
(b) cyanide process
(c) Down process
(d) All of these
Answer:
(a) Castners process Castner’s process
NaOH ⇌ Na+ + OH–
Cathode : Na+ + e– → Na
Anode : 2OH– → H2O + 1/2 O2 + 2e–
Question 11.
The product obtained as a result of a reaction of nitrogen with CaC2 is (NEET – Phase I)
(a) Ca(CN)3
(b) CaN2
(c) Ca(CN)2
(d) Ca3N2
Answer:
(c) Ca(CN)2
Hint:
![]()
Question 12.
Which of the following has highest hydration energy?
(a) MgCl2
(b) CaCl2
(c) BaCl2
(d) SrCl2
Answer:
(a) MgCl2
Hint:
The order of hydration energy of alkaline earth metal is Be2+ > Mg2+ > Ca2+ > Sr2+ > Ba2+
Question 13.
Match the flame colours of the alkali and alkaline earth metal salts in the bunsen burner
(p) Sodium – (1) Brick red
(q) Calcium – (2) Yellow
(r) Barium – (3) Violet
(s) Strontium – (4) Apple green
(t) Cesium – (5) Crimson red
(u) Potassium – (6) Blue
(a) p – 2, q – 1, r- 4, s – 5, t- 6, u – 3
(b) p – 1, q – 2, r – 4, s – 5, t – 6, u – 3
(c) p – 4, q – 1, r – 2, s – 3, t – 5, u – 6
(d) p – 6, q – 5, r – 4, s – 3, t – 1,u – 2
Answer:
(a) p – 2, q – 1, r – 4, s – 5, t – 6, u – 3
(p) sodium – yellow (2)
(p) calcium – brick red (1)
(r) barium – apple green (4)
(s) strontium – crimson red (5)
(t) cesium – blue (6)
(u) potassium – violet (3)
![]()
Question 14.
Assertion : Generally alkali and alkaline earth metals form superoxides Reason : There is a single bond between O and O in superoxides.
(a) both assertion and reason are true and reason is the correct explanation of assertion
(b) both assertion and reason are true but reason is not the correct explanation of assertion
(c) assertion is true but reason is false
(d) both assertion and reason are false
Answer:
(d) both assertion and reason are false
Hint:
Among alkali and alkaline earth metals, K, Rb and Cs alone forms superoxides. Superoxide O2- has 3 electron bond.
Question 15.
Assertion : BeSO4 is soluble in water while BaSO4 is not
Reason: Hydration energy decreases down the group from Be to Ba and lattice energy remains almost constant.
(a) both assertion and reason are true and reason is the correct explanation of assertion
(b) both assertion and reason are true but reason is not the correct explanation of assertion
(c) assertion is true but reason is false
(d) both assertion and reason are false
Answer:
(a) both assertion and reason are true and reason is the correct explanation of assertion
Question 16.
Which is the correct sequence of solubility of carbonates of alkaline earth metals?
(a) BaCO3 > SrCO3 > CaCO3 > MgCO3
(b) MgCO3 > CaCO3 > SrCO3 > BaCO3
(c) CaCO3 > BaCO3 > SrCO3 > MgCO3
(d) BaCO3 > CaCO3 > SrCO3> MgCO3
Answer:
(b) MgCO3 > CaCO3 > SrCO3 > BaCO3
Hint:
Solubility of carbonates decreases down the group.
Question 17.
In context with beryllium, which one of the following statements is incorrect?
(a) It is rendered passive by nitric acid
(b) It forms Be2C
(c) Its salts are rarely hydrolyzed
(d) Its hydride is electron deficient and polymeric
Answer:
(c) Its salts are rarely hydrolyzed
Hint:
Correct statement is beryllium salts are easily hydrolyzed
Question 18.
The suspension of slaked lime in water is known as (NEET Phase – II)
(a) lime water
(b) quick lime
(c) milk of lime
(d) aqueous solution of slaked lime
Answer:
(c) milk of lime
Hint:
Slaked lime Ca(OH)2. The suspension is called milk of lime and the clear solution is called lime water
![]()
Question 19.
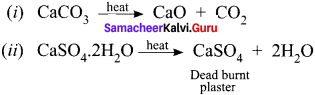
A colourless solid substance (A) on heating evolved CO2 and also gave a white residue, soluble in water. Residue also gave CO2 when treated with dilute HCl.
(a) Na2CO3
(b) NaHCO3
(c) CaCO3
(d) Ca(HCO3)2
Answer:
(b) NaHCO3
Hint:
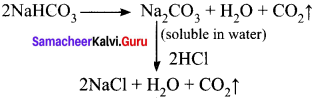
Question 20.
The compound (X) on heating gives a colourless gas and a residue that is dissolved in water to obtain (B). Excess of CO2 is bubbled through aqueous solution of B, C is formed. Solid (C) on heating gives back X. (B) is ………..
(a) CaCO3
(b) Ca(OH)2
(c) Na2CO3
(d) NaHCO3
Answer:
(b) Ca(OH)2
Solution:
![]()
CaO + H2O → Ca(OH)2
Ca(OH)2 + CO2 → CaCO3 + H2O
Question 21.
Which of the following statement is false ? (NEET – Phase -1)
(a) Ca2+ ions are not important in maintaining the regular beating of the heart
(b) Mg2+ ions are important in the green parts of the plants
(c) Mg2+ ions form a complex with ATP
(d) Ca2+ ions are important in blood clotting
Answer:
(a) Ca2+ ions are not important in maintaining the regular beating of the heart
Hint:
Ca2+ ion plays an important role in maintaining regular heart beat.
Question 22.
The name ‘Blue John’ is given to which of the following compounds?
(a) CaH2
(b) CaF2
(c) Ca3(PO4)2
(d) CaO
Answer:
(b) CaF2
Hint:
‘Blue john’ – CaF2 (A variety of fluorite)
Question 23.
Formula of gypsum is ………….
(a) CaSO4.2H2O
(b) CaSO4. ½2H2O
(c) 3CaSO4.H2O
(d) 2CaSO4.2H2O
Answer:
(a) CaSO4.2H2O
Question 24.
When CaC2 is heated in atmospheric nitrogen in an electric furnace the compound formed is
(a) Ca(CN)2
(b) CaNCN
(c) CaC2N2
(d) CaNC2
Answer:
(b) CaNCN
Solution:
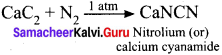
Question 25.
Among the following the least thermally stable is
(a) K2CO3
(b) Na2CO3
(c) BaCO3
(d) Li2CO3
Answer:
(d) Li2CO3
Hint:
Li2CO3 is the least stable.
II. Write a brief answer to the following questions.
Question 26.
Why sodium hydroxide is much more water-soluble than chloride?
Answer:
- Sodium hydroxide is a stronger base whereas sodium chloride is a salt.
- Sodium hydroxide dissolves freely in water with the evolution of much heat on account of intense hydration.
- In other words, when the Na+ and OH– ions break up, the OH– ions are much smaller than Cl– ions and are able to form a hydrogen bond with water.
- Thus sodium hydroxide dissolves easily in water.
![]()
Question 27.
Explain what to meant by efflorescence?
Answer:
- Efflorescence is the formation of powdery deposit on the surface of rock as a result of loss of moisture or water on exposure to air.
- Efflorescence is the formation of whitish powdery deposit on the surface of rocks like gypsum in dry regions. It is formed as mineral-rich water, rises to the surface through capillary action and then evaporates.
- Gypsum crystals are sometimes found to occur in the form that resembles the petals of flower. This happens mostly in arid areas or desert terrains, where there is rapid loss of water. This phenomenon is called as efflorescence.
Question 28.
Write the chemical equations for the reactions involved in Solvay process of preparation of sodium carbonate.
Answer:
Solvay process:
The Solvay process is represented by the below chemical equations:
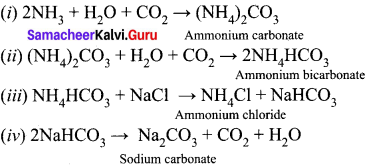
Question 29.
An alkali metal (x) forms a hydrated sulphate, X2SO2. 10H2O. Is the metal more likely to he sodium (or) potassium.
Answer:
Sodium: Because hydration is favoured by high charge density cations and of the two mono positive ions, sodium is smaller and will have higher charge density. Thus, Na2SO4.10H2O is more readily formed.
Question 30.
Write balanced chemical equation for each of the following chemical reactions.
(i) Lithium metal with nitrogen gas
(ii) Heating solid sodium bicarbonate
(iii) Rubidium with oxygen gas
(iv) Solid potassium hydroxide with CO2
(v) Heating calcium carbonate
(vi) Heating calcium with oxygen
Answer:
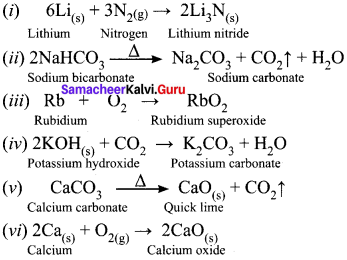
Question 31.
Discuss briefly the similarities between beryllium and aluminium.
Answer:
- Beryllium chloride forms a dimeric structure like aluminium chloride with chloride bridges. Beryllium chloride also forms a polymeric chain structure in addition to the dimer. Both are soluble in organic solvents and are strong Lewis acids.
- Beryllium hydroxide dissolves in excess of alkali and gives beryllate ion and [Be(OH)4]2- and hydrogen as aluminium hydroxide which gives aluminate ion, [Al(OH)4]2-.
- Beryllium and aluminium ions have a strong tendency to form complexes, BeF42-, AlF63-.
- Both beryllium and aluminium hydroxides are amphoteric in nature.
- Carbides of beryllium (Be2C) like aluminium carbide (Al4C3) give methane on hydrolysis.
- Both beryllium and aluminium are rendered passive by nitric acid.
Question 32.
Give the systematic names for the following:
- milk of magnesia
- lye
- lime
- caustic potash
- washing soda
- soda ash and
- trona.
Answer:
- Milk of magnesia – Mg(OH)2 – Magnesium hydroxide
- Lye – NaOH – Sodium hydroxide
- Lime – Ca(OH)2 Calcium hydroxide
- Caustic potash – KOH – Potassium hydroxide
- Washing soda – Na2CO3. 10H2O – Sodium carbonate decahydrate
- Soda ash – Na2CO3 – Sodium carbonate (anhydrous)
- Trona – NaCO3.NaHCO3.2H2O – Sodium sesqui carbonate
Question 33.
Substantiate Lithium fluoride has the lowest solubility among group one metal fluorides.
Answer:
Lithium fluoride has the lowest solubility among alkali metal fluoride due to its small size of Li+ and F– ions, lattice enthalpy is much higher than that of hydration enthalpy.
Question 34.
Mention the uses of Plaster of Paris.
Answer:
- The largest use of Plaster of Paris is in the building industry as well as plasters.
- It is used for immobilizing the affected part of organ, where there is a bone fracture or sprain.
- It is also employed in dentistry, in ornamental work and for making casts of statues and busts.
Question 35.
Beryllium halides are covalent whereas magnesium halides are ionic why?
Answer:
Halogens are non-metals and beryllium is also non-metal. Since non-metals always form covalent bonds with each other due to almost similar ionization potential and electronegativity. And Beryllium is smaller in size and has high polarizing power therefore, beryllium halides are covalent.
Magnesium is a metal and metals mostly form ionic bonds with non-metals due to vast differences in their ionization potential and electronegativity, therefore magnesium halides are always ionic.
![]()
Question 36.
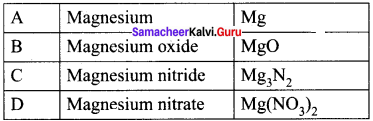
Alkaline earth metal (A), belongs to 3rd period reacts with oxygen and nitrogen to form compound (B) and (C) respectively. It undergo metal displacement reaction with AgNO3 solution to form compound (D).
Answer:
- An alkaline earth (A) metal belongs to third period is magnesium (Mg).
- Magnesium reacts with oxygen to form magnesium oxide (MgO) (B).

- Magnesium reacts with nitrogen to form magnesium nitride Mg3N2 (C).
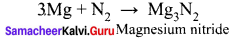
- Magnesium undergoes metal displacement reaction with AgNO3 solution to form magnesium nitrate Mg(NO3)3 (D).

Question 37.
Write balanced chemical equation for the following processes:
(a) heating calcium in oxygen
(b) heating calcium carbonate
(c) evaporating a solution of calcium hydrogen carbonate
(d) heating calcium oxide with carbon
Answer:
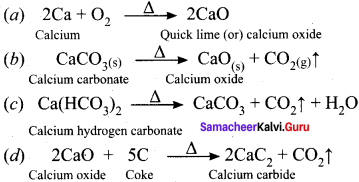
Question 38.
Explain the important common features of group 2 elements. Important common features of group 2 elements.
Answer:
- Group 2 is known as alkaline earth metals. It contains soft, silver metals that are less metallic in character than the Group 1 elements. Although many characteristics are common throughout the group, the heavier metals such as Ca, Sr, Ba, and Ra are almost as reactive as the Group 1 Alkali Metals.
- General electronic configuration can be represented as [Noble gas] ns2 where ‘n’ represents the valence shell.
- All the elements in Group 2 have two electrons in their valence shells, giving them an oxidation state of +2. This enables the metals to easily lose electrons, which increases their stability and allows them to form compounds via ionic bonds.
- The atomic and ionic radii of alkaline earth metals are smaller than the corresponding members of the alkali metals.
- On moving down the group, the radii increases due to gradual increase in the number of the shells and the screening effect.
- Down the group the ionisation enthalpy decreases as atomic size increases. They are less electropositive than alkali metals.
- Compounds of alkaline earth metals are more extensively hydrated than those of alkali metals because the hydration enthalpies of alkaline earth metal ions are larger than those of alkali metal ions.
Question 39.
Discuss the similarities between beryllium and aluminium.
Answer:
Similarities between Beryllium and Aluminium:
- Beryllium chloride forms a dimeric structure like aluminium chloride with chloride bridges. Beryllium chloride also forms a polymeric chain structure in addition to the dimer. Both are soluble in organic solvents and are strong Lewis acids.
- Beryllium hydroxide dissolves in excess of alkali and gives beryllate ion and [Be(OH)4]2-and hydrogen as aluminium hydroxide which gives aluminate ion, [Al(OH)4]–.
- Beryllium and aluminium ions have a strong tendency to form complexes, BeF42-, AlF63-.
- Both beryllium and aluminium hydroxides are amphoteric in nature.
- Carbides of beryllium (Be2C) like aluminium carbide (Al4C3) give methane on hydrolysis.
- Both beryllium and aluminum are rendered passive by nitric acid.
Question 40.
Why alkaline earth metals are harder than alkali metals?
Answer:
1. The strength of metallic bond in alkaline earth metals is higher than alkali metals due to the presence of 2 electrons in its outermost shell as compared to alkali metals, which have only 1 electron in valence shell. Therefore, alkaline earth metals are harder than alkali metals.
2. The alkaline earth metals have greater nuclear charge and more valence electrons, thus metallic bonding is more effective. Due to this they are harder than alkali metals.
Question 41.
How is plaster of paris prepared?
Answer:
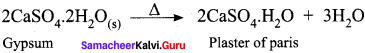
Plaster of paris is a hemihydrate of calcium sulphate CaSO4. H2O. It is obtained by heating gypsum at 393 K.
Question 42.
Give the uses of gypsum.
Answer:
- Gypsum is used in making drywalls or plasterboards. Plasterboards are used as the finish for walls and ceilings, and for partitions.
- Another important use of gypsum in the production of plaster of Paris. Gypsum is heated to about 300 degrees Fahrenheit to produce plaster of Paris, which is also known as gypsum plaster. It is mainly used as a sculpting material.
- Gypsum is used in making surgical and orthopedic casts, such as surgical splints and casting moulds.
- Gypsum plays an important role in agriculture as a soil additive, conditioner, and fertilizer. It helps loosen up compact or clay soil and provides calcium and sulphur, which are essential for the healthy growth of a plant. It can also be used for removing sodium from soils having excess salinity.
- Gypsum is used in toothpaste, shampoos, and hair products, mainly due to its binding and thickening properties.
- Gypsum is a component of Portland cement, where it acts as a hardening retarder to control the speed at which concrete sets.
Question 43.
Describe briefly the biological importance of calcium and magnesium.
Answer:
- An adult body contains about 25 g of Mg and 1200 g of Ca. The daily requirement in the human body has been estimated to be 200-300 mg.
- Magnesium is the co-factor of all enzymes that utilize ATP in phosphate transfer and energy release.
- The main pigment for the absorption of light in plants is chlorophyll which contains magnesium.
- About 99% of body calcium is present in bones and teeth.
- Calcium plays important roles in neuromuscular function, interneuronal transmission, cell membrane integrity and blood coagulation.
- The calcium concentration in plasma is regulated at about 100 mgL-1. It is maintained by two hormones: calcitonin and parathyroid hormone.
- Deficiency of magnesium results into convulsion and neuromuscular irritation.
- 2% of adult weight is made up of calcium. Calcium phosphate is present in teeth and Calcium carbonate is present in bones. They make the teeth and bone hard.
- Water in the human body such as inside the cell and in the blood contain dissolved calcium ions. These ions are involved in making muscles move and in sending electricity around the brain and along the nerves.
- Magnesium is an essential element in both plant and animal life.
Question 44.
Which would you expect to have a higher melting point, magnesium oxide or magnesium fluoride? Explain your reasoning.
Answer:
- Magnesium fluoride – 1263°C
- Magnesium oxide – 2852°C
- The strength of ionic bonds usually depends on two factors – ionic radius and charge. Mg2+ and O2- have charges of +2 and -2 respectively. This is larger than the charge of other ions.
- Magnesium ions and oxygen ions also have a small ionic radius.
- Oxygen ion is smaller than fluoride
- The smaller the ionic radii, the smaller the bond length and the stronger the bond. Therefore the ionic bond between magnesium and oxygen is very strong.
Samacheer Kalvi 11th Chemistry Alkali and Alkaline Earth Metal Additional Questions Solved
I. Choose the correct answer
Question 1.
The reducing property of alkali metals follows the order
(a) Na < K < Rb < Cs < Li
(b) K < Na < Rb < Cs < Li
(c) Li < Cs < Rb < K < Na
(d) Rb < Cs < K < Na < Li
Answer:
(a) Na < K < Rb < Cs < Li
Question 2.
The general electronic configuration of alkali metals is ………….
(a) [noble gas] ns2
(b) [noble gas] ns1
(c) ns2 np6
(d) ns2(n-1)d1-10
Answer:
(b) [noble gas] ns1
Question 3.
Li does not resemble other alkali metals in which of the following property?
(a) Li2CO3 decomposes into oxides while other alkali carbonates re thermally stable
(b) LiCl is predominantly covalent
(c) Li3N stable
(d) All of the above
Answer:
(d) All of the above
Question 4.
The half-life period of francium is ………….
(a) 21 days
(b) 21 years
(c) 2.1 minutes
(d) 21 minutes
Answer:
(d) 21 minutes
Question 5.
The alkali metal used in photoelectric cells is
(a) Na
(b) Cs
(c) Rb
(d) Fr
Answer:
(b) Cs
![]()
Question 6.
The metal present in deposits of nitre is ……………
(a) lithium
(b) potassium
(c) rubidium
(d) francium
Answer:
(b) potassium
Question 7.
Be2C + 4H2O → 2X + CH4
X + 2HCl + 2H2O → Y
X and Y formed in the above two reactions is
(a) BeCO3 and Be(OH)2 respectively
(b) Be(OH)2 and BeCl2 respectively
(c) Be(OH)2 and [Be(OH)4]Cl2 respectively
(d) [Be(OH)4]2- and BeCl2 respectively
Answer:
(c) BQ(OH)2 and [Be(OH)4]Cl2 respectively
Question 8.
Which of the following are stored under oil?
(a) Alkali metals
(b) Coinage metals
(c) Noble metals
(d) Phosphorous
Answer:
(a) Alkali metals
Question 9.
Magnesium burns in the air to give
(a) MgO
(b) MgCO3
(c) MgCO3
(d) MgO and Mg3N2
Answer:
(d) MgO and Mg3N2
Question 10.
The most common oxidation state of alkali metals is ………….
(a) +1
(b) +2
(c) +3
(d) +5
Answer:
(a) +1
Question 11.
The word ‘alkali’ used for alkali metals indicates
(a) ashes of plants
(b) metallic luster
(c) soft metals
(d) reactive metals
Answer:
(a) ashes of plants
Question 12.
Which of the following salt is more soluble?
(a) NaClO4
(b) LiClO4
(c) CsBr
(d) KI
Answer:
(b) LiClO4
Question 13.
Select the correct statement?
LiOH > NaOH > KOH > RbOH
Li2CO3 > Na2CO3 > K2CO3 > Rb2CO3
(a) Solubility of alkali hydroxides is in order
(b) Solubility of alkali carbonates is in order
(c) both are correct
(d) None is correct
Answer:
(b) Solubility of alkali carbonates is in order
Question 14.
Which one of the following is less soluble in water?
(a) LiC
(b) NaCl
(c) KCl
(d) CsI
Answer:
(a) LlCl
Question 15.
Which of the following ions form a hydroxide highly soluble in water?
(a) Ni2+
(b) K2+
(c) Zn2+
(d) Al3+
Answer:
(b) K2+
Question 16.
Consider the following statements.
(1) Lithium does not have d-orbitais.
(ii) Lithium carbonate is more soluble than sodium carbonate in water.
(iii),The second ionization enthalpy of alkali metals are zero.
Which of the above statements is/are not correct?
(a) (i) only
(b) (ii) only
(c) (ii) and (iii)
(d) (i), (ii) and (iii)
Answer:
(c) (ii) and (iii)
![]()
Question 17.
The carbide of which of the following metals on hydrolysis gives allylene or propyne?
(a) Be
(b) Ca
(c) Al
(d) Mg
Answer:
(d) Mg
Question 18.
Which colour is produced when alkali metals dissolved in liquid ammonia?
(a) Red
(b) Green
(c) Blue
(d) Violet
Answer:
(c) Blue
Question 19.
Consider the following statements.
(i) Superoxides of alkali metals are diamagnetic.
(ii) Superoxides of alkali metals are blue in colour.
(iii) Superoxides of alkali metals are paramagnetic.
Which of the above statements is/are not correct?
(a) (i) only
(b) (ii) only
(c) (iii) only
(d) (i) and (ii)
Answer:
(d) (i) and (ii)
Question 20.
Which of the following is known as a variety of gypsum?
(a) CaCO3
(b) CaSO4
(c) plaster of paris
(d) gypsum
Answer:
(d) gypsum
Question 21.
The colour produced by potassium when burnt in Bunsen flame is …………
(a) red
(b) blue
(c) green
(d) lilac
Answer:
(d) lilac
Question 22.
Which one of the following is a radioactive element of alkali metal?
(a) Cesium
(b) Francium
(c) Potassium
(d) Sodium
Answer:
(b) Francium
Question 23.
Which of the following ions perform important biological functions in maintenance of the ion balance and nerve impulse conduction?
(a) Li+, Rb+
(b) Na+, K+
(c) Cs+,Fr+
(d) Rb+, Cs+
Answer:
(b) Na+, K+
Question 24.
The colour of potassium salt in flame is
(a) Crimson red
(b) Lilac
(c) Blue
(d) Yellow
Answer:
(b) Lilac
Question 25.
Which of the following ions arc more responsible for transmission of nerve signal?
(a) Li+
(b) Rb+
(c) Cs+
(d) K+
Answer:
(d) K+
Question 26.
Which of the following fruits contain maximum of potassium?
(a) Grapes
(b) Potatoes
(c) Bananas
(d) Mangoes
Answer:
(c) Bananas
Question 27.
Which of the following element forms monoxide and peroxide?
(a) Lithium
(b) Potassium
(c) Rubedium
(d) Sodium
Answer:
(d) Sodium
Question 28.
Among the following, which is the fifth most abundant element?
(a) Beryllium
(b) Barium
(c) Radium
(d) Calcium
Answer:
(d) Calcium
![]()
Question 29.
Celestite and strontianite are the ores of ………..
(a) cesium
(b) strontium
(c) magnesium
(d) barium
Answer:
(b) strontium
Question 30.
The products obtained on the reaction of Na2O2 with water are
(a) NaOH and H2O
(b) NaOH and H2O2
(c) Na2O and H2O2
(d) NaOH, Na2O
Answer:
(c) Na2O and H2O2
Question 31.
Which one of the following gives green spark in fire works?
(a) Magnesium chloride
(b) Sodium chloride
(c) Barium bromide
(d) Potassium iodide
Answer:
(a) Magnesium chloride
Question 32.
Statement – 1:
LiF has low solubility in water.
Statement – 2:
LiF has low lattice enthalpy.
In the above statements
(a) 1 alone is correct.
(b) Both 1 and 2 are correct
(c) 2 alone is correct
(d) Both 1 and 2 are incorrect
Answer:
(b) Both 1 and 2 are correct
Question 33.
Copper chloride produces colour in fire works.
(a) red
(b) green
(c) blue
(d) yellow
Answer:
(c) blue
Question 34.
The ammonia used in the Solvay proves- recovered by using
(a) calcium chloride
(b) Calcium hydroxide
(c) calcium carbonate
(d) calcium oxide
Answer:
(b) Calcium hydroxide
Question 35.
The most common oxidation state of alkaline earth metals is ……………
(a) +4
(b) +2
(c) + 1
(d) +3
Answer:
(b) +2
Question 36.
Sodium carbonate decahydrate on heating ab 373K gives
(a) Na2CO3 .3H2O
(b) N2CO3 .5H2O
(c) Na2CO3
(d) Na2CO3 .H2O
Answer:
(c) Na2CO3
![]()
Question 37.
Hydroxides of beryllium are in nature.
(a) neutral
(b) basic
(c) acidic
(d) amphoteric
Answer:
(d) amphoteric
Question 38.
The product obtained on saturating a solution o sodium carbonate with carbon dioxide i,-.
(a) sodium bicarbonate
(b) sodium hydroxide
(c) sodium chloride
(d) sodium peroxide.
Answer:
(a) sodium bicarbonate
Question 39.
Which one of the following alkaline earth metal is not readily attacked by acids?
(a) Magnesium
(b) Calcium
(c) Beryllium
(d) Strontium
Answer:
(c) Beryllium
Question 40.
The used in baking cakes, pastries, etc., is
(a) sodium chloride
(b) sodium carbonate
(c) sodium bicarbonate
(d) sodium hydroxide
Answer:
(c) sodium bicarbonate
Question 41.
Which one of the following is used to build the beam pipe in accelerators?
(a) Be
(b) Ca
(c) Mg
(d) Sr
Answer:
(a) Be
Question 42.
Fluorapatite is the ore of
(a) magnesium
(b) beryllium
(c) potassium
(d) calcium
Answer:
(d) calcium
Question 43.
Which metal is used in photoengrave plates in printing industry?
(a) Co
(b) Pt
(c) Zn
(d) Mg
Answer:
(d) Mg
Question 44.
Strontium nitrate give _______ colour in fire works.
(a) violet
(b) bright red
(c) green
(d) orange
Answer:
(b) bright red
Question 45.
Which is used in dehydrating oils?
(a) Calcium
(b) Magnesium
(c) Beryllium
(d) Radium
Answer:
(a) Calcium
![]()
Question 46.
Correctly match List-I and List-II using the code given below the list.
List-I
A. Beryllium
B. Calcium
C. Magnesium
D. Barium
List-II
1. Sacrificial anode
2. X-ray tube radiation window
3. Scavenger to remove oxygen in TV
4. Getter in vacuum tubes

Answer:
![]()
Question 47.
Correctly match List-I and List-II using the code given below the list.
List-I
A. Beryllium
B. Magnesium
C. Calcium
D. Strontium
List-II
1. Cement
2. Dating of rocks
3. X-ray detector
4. Missile construction

Answer:
![]()
Question 48.
Correctly match the list-I and List-II using the code given below the list.
List-I
A. Radium
B. Barium
C. Strontium
D. Calcium
List-II
1. Dehydration of oils
2. Aircraft and watches
3. Deoxidiser in copper refining
4. Radioactive tracer

Answer:
![]()
Question 49.
Consider the following statements.
(i) BeO is basic.
(ii) MgO is weakly basic.
(iii) BaO is strongly acidic.
Which of the above statements is/are not correct?
(a) (i) only
(b) (ii) only
(c) (ii) and (iii)
(d) (i) and (iii)
Answer:
(d) (i) and (iii)
Question 50.
______ is a major component of bones and teeth.
(a) Na
(b) Be
(c) Ca
(d) Mg
Answer:
(c) Ca
![]()
Question 51.
Correctly match the list-I and List-II using the code given below the list.
List-I
A. Quick lime
B. Calcium hydroxide
C. Gypsum
D. Plaster of Paris
List-II
1. Casts of statues
2. Drying agent
3. White washing
4. Tooth paste

Answer:
![]()
Question 52.
Correctly match the list-I and List-II using the code given below the list.
List-I
A. CaO
B. Ca(OH)2
C. CaSO4 .2H2O
D. CaSO4 .½2H2O
List-II
1. Plaster of Paris
2. Quick lime
3. Slaked lime
4. Gypsum

Answer:
![]()
Question 53.
Which one of the following is the formula of limestone?
(a) CaO
(b) Ca(OH)2
(c) CaCO3
(d) CaCO3.MgCO3
Answer:
(c) CaCO3
Question 54.
Which one of the following is used in purification of sugar and as drying agent?
(a) Ca(OH)2
(b) MgSO4.7H2O
(c) CaSO4.2H2O
(d) CaO
Answer:
(d) CaO
Question 55.
Which one of the following is named as bleaching powder?
(a) CaCl2
(b) CaOCl
(c) Ca(OCl)2
(d) Ca(HCO3)2
Answer:
(c) Ca(OCl)2
Question 56.
Which one of the following is known as natural insulator?
(a) FeSO4.7H2O
(b) NaCO4.10H2O
(c) CaSO4.2H2O
(d) CaSO4.’/2H2O
Answer:
(c) CaSO4.2H2O
![]()
Question 57.
Which one of the following is called ornamental stone?
(a) Alabaster
(b) Plaster of Paris
(c) Limestone
(d) Gypsum plaster
Answer:
(a) Alabaster
Question 58.
Which one of the following is used in toothpaste, shampoo and hair products?
(a) Plaster of Paris
(b) Limestone
(c) Quick lime
(d) Gypsum
Answer:
(d) Gypsum
Question 59.
Which one of the following plays an important role in agriculture as a soil additive, conditioner and fertilizer?
(a) Epsurn
(b) Gypsum
(c) Quick lime
(d) Salt petre
Answer:
(b) Gypsum
Question 60.
Which is used to treat upset stomach and eczema?
(a) MgSO4.7H2O
(b) FeSO4.7H2O
(c) CaSO4.2H2O
(d) 2CaSO4H2O
Answer:
(c) CaSO4.2H2O
Question 61.
Consider the following statements.
(i) Gypsum is used in making surgical and orthopedic casts.
(ii) Calcium nitrate acts a coagulator in making tofu.
(iii) Gypsum plays an important role in soap making.
Which of the above statements is/are not correct.
(a) (i) only
(b) (ii) and (iii)
(c) (i) and (iii)
(d) (ii) only
Answer:
(b) (ii) and (iii)
Question 62.
About 393K, when Plaster of Paris is heated, it forms
(a) burnt alum
(b) dead burnt plast
(c) gypsum plaster
(d) alabaster
Answer:
(b) dead burnt plast
Question 63.
Which of the following is used in dentistry, ornamental works and making casts of statues?
(a) CaSO4.2H2O
(b) CaSO4.½H2O
(c) CaO
(d) Ca(OH)2
Answer:
(b) CaSO4.½H2O
Question 64.
Which one of the following metal act as co-factor in phosphate transfer of ATP by enzymes?
(a) Calcium
(b) Beryllium
(c) Magnesium
(d) Sodium
Answer:
(c) Magnesium
![]()
Question 65.
The main pigment in plants is chlorophyll which contains ……………
(a) iron
(b) calcium
(c) barium
(d) magnesium
Answer:
(d) magnesium
Question 66.
Consider the following statements.
(i) 99% of body calcium is present in bones and teeth.
(ii) The calcium concentration in plasma is regulated at 10 mg L-1.
(iii) Calcium plays an important role in neuromuscular function, interneuronal transmission and in blood coagulation.
Which of the above statements is/are not correct?
(a) (i) only
(b) (ii) only
(c) (iii) only
(d) (i), (ii) and (iii)
Answer:
(b) (ii) only
Question 67.
Correctly match the list-I and list-Il using the code given below the list,
List-I
A. Chlorophyll
B. Bones
C. Dentistry
D. Cement
List-II
1. Plaster of paris
2. Gypsum
3. Magnesium
4. Calcium.

Answer:
![]()
Question 68.
Which one of the following is the most common alkaline earth metal found in the human body?
(a) Beryllium
(b) Magnesium
(c) Barium
(d) Calcium
Answer:
(d) Calcium
Question 69.
which alkaline earth metal do not import colour to a non-luminous flame?
(a) Beryllium
(b) Calcium
(c) Magnesium
(d) Barium
Answer:
(a) Beryllium
Question 70.
Statement-I: Alkali metals arc very soft metals.
Statement-II: Since the atoms of alkali metals have bigger kernels and smaller number ot valence electrons, the metallic bonds in them are very weak and hence they arc soft.
(a) Statements-I and II arc correct but statement-II is not the correct explanation of statement-I.
(b) Statements-I and II are correct and statement-II is the correct explanation of statement-I.
(c) Statement-I is correct but statement-lI is wrong.
(d) Statement-I is wrong but statement-II is correct.
Answer:
(b) Statements-I and II are correct and statement-II is the correct explanation of statement-I.
Question 71.
Statement-I: BeCl2 is soluble in organic solvent.
Statement-II: Since BeCl2 is a covalent compound, it is soluble in organic solvent.
(a) Statements-I and II arc correct and statement-II is the correct explanation of statement-I.
(b) Statements-I and II are correct but statement-II is not the correct explanation of statement-I.
(c) Statement-I is wrong but statement II is correct.
(d) Statement-I is correct but statement-II is wrong.
Answer:
(a) Statements-I and II are correct and statement-II is the correct explanation of statement-I.
![]()
Question 72.
Which one of the following is more basic?
(a) Ca(OH)2
(b) Mg(OH)2
(c) NaOH
(d) Al(OH)3
Answer:
(c) NaOH
Question 73.
Statement-I: Cesium is considered as the most electropositive element.
Statement-II: Due to its lowest ionization energy, cesium is considered as the most electropositive element.
(a) Statements-I and II are correct and statement-II is the correct explanation of statemcnt-I.
(b) Statements-I and II are correct but statement-II is not the correct explanation of statement-I.
(c) Statement-I is correct but statement-II is wrong.
(d) Statement-I is wrong but statement-II is correct.
Answer:
(a) Statements-I and II are correct and statement-II is the correct explanation of statement-I.
Question 74.
The reducing property of alkali metals follows the order
(a) Na<K<Rb<Cs<Li
(b) K<Na<Rb<Cs<Li
(c) Li<Cs<Rb<K<Na
(a) Rb<Cs<K<Na<Li
Answer:
(a) Na<K<Rb<Cs<Li
Question 75.
Which of the following is the least thermally stable?
(a) MgCO3
(b) CaCO3
(c) SrCO3
(d) BeCO3
Answer:
(d) BeCO3
Question 76.
Which of the following is not a peroxide?
(a) KO2
(b) CrO5
(c) Na2O2
(d) BaO2
Answer:
(a) KO2
Question 77.
Which of the following is used in photoelectric cells?
(a)Na
(b) K
(e) Li
(d) Cs
Answer:
(d) Cs
Question 78.
When caesìum salt is subjected to flame test, the colour produced is
(a) lilac
(b) yellow
(c) blue
(d) crimson red
Answer:
(c) blue
Question 79.
Match the list-I and List-II using the correct code given below the list.
List-I
A. Lithium
B. Sodium
C. Potassium
D. Rubidium
List-II
1. Lilac
2. Reddish yiolet
3. Crimson red
4. Yellow

Answer:
![]()
Question 80.
Which of the following alloy is used in making white metal bearings for motor engines?
(a) Lithium + magnesium
(b) Lithium + lead
(c) Lithium + aluminium
(d) Lithium + copper
Answer:
(b) Lithium + lead
![]()
Question 81.
Lithium aluminium alloy is used in making
(a) armour plates
(b) white metal bearings
(c) electrochemical cell
(d) aircraft parts
Answer:
(d) aircraft parts
Question 82.
Which of the following is used in making armour plates?
(a) Lithium + magnesium
(b) Lithium + aluminium
(c) Lithium + lead
(d) Sodium + lithium
Answer:
(a) Lithium + magnesium
Question 83.
Which metal is used in making electrochemical cells?
(a) Caesium
(b) Lithium
(c) Calcium
(d) Barium
Answer:
(b) Lithium
Question 84.
Which of the following is used as a coolant in fast breeder nuclear reactor?
(a) Liquid ammonia
(b) Liquid helium
(c) Liquid Na metal
(d) Solid CO2
Answer:
(c) Liquid Na metal
Question 85.
Which of the following is an excellent absorbent of carbon dioxide?
(a) K2 CO3
(b) CaCO3
(c) NaOH
(d) KOH
Answer:
(d) KOH
Question 86.
Which of the following is used in devising photoelectric cells?
(a) Li
(b) Cs
(c) Na
(d) K
Answer:
(b) Cs
Question 87.
The formula of washing soda is ……………..
(a) Na2 CO3
(b) NaHCO3
(c) Na,CO3 .10H2 O
(d) Ca(HCO3)2
Answer:
(c) Na2CO3 .10H2O
Question 88.
Match the list-I and List-II using the correct code given below the list.
List-I
A. Na2CO3
B. Na2CO3 .10H2O
C. NaHCO3
D. NaOH
List-II
1. Caustic soda
2. Baking soda
3. Soda ash
4. Washing soda

Answer:
![]()
Question 89.
Which one of the following is used for mercerising cotton fabrics?
(a) KOH
(b) NaOH
(c) Na2CO3
(d) NaHCO3
Answer:
(b) NaOH
Question 90.
Which one of the following is used in fire extinguishers?
(a) Washing soda
(b) Soda ash
(c) Baking soda
(d) Caustic soda
Answer:
(c) Baking soda
Question 91.
Assertion (A): Sodium hydrogen carbonate is used in baking cakes and pastries.
Reason (R): On heating sodium hydrogen carbonate, liberates bubbles of CO2 leaving holes in cakes and making them light and fluffy.
(a) both (A) and (R) are correct and (R) is the correct explanation of (A)
(b) both (A) and (R) are correct but (R) is not the correct explanation of(A)
(c) (A) is correct but (R) is wrong
(d) (A) is wrong but (R) is correct
Answer:
(a) both (A) and (R) are correct and (R) is the correct explanation of (A)
Question 92.
Match the List-I and List-II using the correct code given below the list.
List-I
A. Manufacture of soap
B. Mild antiseptic
C. Softening of hard water
D. Coolant in nuclear reactor
List-II
1. Na2 CO3 . 10H2 O
2. Liquid Na metallic
3. NaOH
4. NaHCO3

Answer:
![]()
Samacheer Kalvi 11th Chemistry Alkali and Alkaline Earth Metal 2-Mark Questions
Question 1.
What are s-block elements?
Answer:
The elements belonging to the group 1 and 2 in the modem periodic table are called s-block elements. The elements belonging to these two groups are commonly known as alkali and alkaline earth metals respectively.
Question 2.
Why group 1 elements are called alkali metals?
Answer:
Group I elements form strong hydroxides on reaction with water which are strong alkaline in nature. So, group 1 elements are called alkali metals.
Question 3.
Why does the ionization enthalpy of alkali metals decreases in a group?
Answer:
Alkali metals have the lowest ionisation enthalpy compared to other elements present in the respective period. As we go down the group, the ionisation enthalpy decreases due to the increase in atomic size. In addition, the number of inner shells also increases, which in turn increases the magnitude of screening effect and consequently, the ionisation enthalpy decreases down the group.
![]()
Question 4.
Alkali metals are stored under oil. Give reason.
Answer:
Alkali metals are so reactive and they have to be stored under oil. Because when they are kept in air, they will bum immediately.
Question 5.
Name the list of elements present in alkali metal group. What is the general electronic configuration of them?
Answer:
Lithium, sodium, potassium, rubidium, caesium and francium are the elements present in alkali metal group. Their general electronic configuration is [noble gas] ns1 .
Question 6.
Why is lithium salts are more soluble than the salts of other metals of group-1?
Answer:
Lithium salts are more soluble than the salts of other metals of group 1. eg., LiClO4 is up to 12 times more soluble than NaClO4. KClO4, RbClO4, and CsClO4 have solubilities only 10-3 times that of LiClO4. The high solubility of Li salts is due to strong solvation of small size of Li+ ion.
Question 7.
The second ionization enthalpy of alkali metals are very high. Give reason.
Answer:
The removal of one electron from the alkali metals causes the formation of monovalent cations having very stable electronic configuration. Therefore it becomes very difficult to remove the second electron from the stable noble gas configuration, giving rise to very high second ionization energy values.
Question 8.
Why does the solubility of carbonates and bicarbonates decrease in a group?
Answer:
All the carbonates and bicarbonates are soluble in water and their solubilities increase rapidly on descending the group. This is due to the reason that lattice energies decrease more rapidly than their hydration energies on moving down the group.
Question 9.
Why lithium has anomalous behaviour than other elements ¡n the same group?
Answer:
The anomalous behaviour of lithium is due to the exceptionally small size of the atom and high polarizing power, which is a ratio of charge to radius and hydration energy.
Question 10.
What is washing soda?
Answer:
Sodium carbonate, commonly known as washing soda, crystallizes as decahydrate which is white in colour.
Question 11.
Lithium forms monoxide with oxygen whereas sodium forms peroxide with oxygen. Why?
Answer:
- The fact that a small cation can stabilize a small anion and a large cation can stabilize a large anion explains the formation and stability of the oxides.
- The size of Li+ ion is very small and it has a strong positive field around it. It can combine with only small anion, O2- ion, resulting in the formation of monoxide Li2O.
- The Na ion is a larger cation and has a weak positive field around it and can stabilize a bigger peroxide ion, O22- or [-O-O-]2- resulting in the formation of peroxide Na2O2.
Question 12.
How does Lithium show similar properties with magnesium in its chemical behavior?
Answer:
- Both react with nitrogen to form nitrides.
- Both react with oxygen to give monoxides.
- Both the element have a tendency to form covalent compounds.
- Both can form complex compounds.
Question 13.
Alkali metal hydrides are strong reducing agents. Prove this statement.
Answer:
The decrease in ionization enthalpy down the group permits easy availability of electrons to forms H ions. So, the hydrides behave as reducing agent. Their reducing nature increases down the group.
![]()
Question 14.
Write the chemical formula of the following compounds.
(a) Chile saltpeter
(b) marble
(c) Brine
Answer:
(a) Chile salt petre – NaNO3
(b) marble – CaCO3
(c) Brine – NaCl
Question 15.
Explain the action of sodium with water.
Answer:
Sodium reacts so rapidly with water with the evolution of heat. The metal whizzes around the surface of water. The hydrogen gas liberated may catch fire giving yellow coloured flame
because of sodium.
2Na + 2H2O → 2NaOH + H2↑ + heat
Question 16.
(a) Lithium Iodide is more covalent than Lithium fluoride
(b) Lattice enthalpy of LiF is maximum among all the alkali metals halides. Explain.
Answer:
(a) According to Fagan’s rule, Li+ ion can polarise I– ion more than the F– ion due to the bigger size of the anion. Thus, LiI has a more covalent character than LiF.
(b) Smaller the size (internuclear distance), more is the value of Lattice enthalpy since the internuclear distance is expected to be least in the LiF.
Question 17.
LiCl is soluble in water whereas LiBr and LiI are soluble In organic solvent. Give reason.
Answer:
Lithium chloride (LiCl) is ionic in nature and it is soluble in polar solvent water, whereas lithium bromide and lithium iodide are covalent and are soluble in non-polar organic solvents.
Question 18.
Give two uses of alkali metals.
Answer:
- Lithium metal is used to make useful alloys. For example, lead is used to make ‘white metal’ bearings for motor engines, with aluminium to make aircraft parts, and with magnesium to make armour plates. It is used in thermonuclear reactions.
- Lithium is also used to make electrochemical cells.
Question 19.
What are the elements present in group 2? Give their general electronic configuration.
Answer:
Group 2 contains beryllium, magnesium, calcium, strontium, barium and radium. They’re general electronic configuration is [noble gas] ns2.
Question 20.
What are alkaline earth metals?
Answer:
Group 2 in the modem periodic table contains the elements beryllium, magnesium, calcium, strontium, barium and radium are called alkaline earth metals.
Question 21.
Atomic radii of alkaline earth metals are smaller than the corresponding members of alkali metals. Why?
Answer:
The atomic radii of alkaline earth metals are smaller than alkali metals. This is due to the fact that group 2 elements have a higher nuclear charge, allowing the electrons to move towards the nucleus. This reduces the size of atomic and ionic radii.
Question 22.
Write the uses of Beryllium.
Answer:
- Because of its low atomic number and very low absorption for X-rays, it is used as radiation windows for X-ray tubes and X-ray detectors.
- The sample holder in X-ray emission studies usually made of beryllium
- Since beryllium is transparent to energetic particles it is used to build the ‘beam pipe’ in accelerators.
- Because of its low density and diamagnetic nature, it is used in various detectors.
Question 23.
Explain the action of halogen with alkaline earth metals.
Answer:
All the alkaline earth metals combine with halogen at elevated temperature to form their halides.
M + X2 → MX2, Where M = Be, Mg,Ca,Sr,Ba and Ra. X= F,Cl,Br and I.
For e.g., Be + Cl2 → BeCl2.
Question 24.
How beryllium chloride is prepared from beryllium oxide?
Answer:
![]()
Beryllium oxide is heated with carbon and chloride to get BeCl2.
Question 25.
How does beryllium hydride can be prepared?
Answer:
All the elements except beryllium, combine with hydrogen on heating to form their hydrides with general formula MH2. BeH2 can be prepared by the reaction of BeCl2 with LiAlH4.
2BeCl2 + LiAlH4 → 2BeH2 + LiCl + AlCl3
Question 26.
Mention the uses of beryllium.
Answer:
- Beryllium is used as radiation windows for X-ray tubes and X-ray detectors.
- The sample holder in X-ray emission studies is made of beryllium.
- Beryllium is used to build the beam pipe in accelerators.
- Beryllium is used in detectors due to its low density and diamagnetic nature.
Question 27.
Write about the uses of strontium.
Answer:
- 90Sr is used in cancer therapy.
- 87Sr / 86Sr ratio is used in marine investigators as well as in teeth, tracking animal migrations or in criminal forensics.
- Dating of rocks.
- Strontium is used as a radioactive tracer in determining the source of archaeological materials such as timbers and coins.
Question 28.
Mention the uses of radium.
Answer:
- In self-luminous paints for watches.
- In nuclear panels.
- In aircraft switches.
- In clocks and instrument dials.
Question 29.
BeO is covalent where as MgO is ionic. Give reason.
Answer:
Beryllium oxide (BeO) is covalent due to the small size of Be2+ ion, while magnesium oxide (MgO) is ionic due to the bigger size of Mg2+ ion.
Question 30.
How is barium peroxide prepared?
Answer:
Barium peroxide is prepared by heating monoxides with oxygen at high temperature.
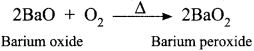
Question 31.
How would you prepare quick lime?
Answer:
Quick lime is produced on commercial scale by heating limestone in a lime kiln at 1173K.
![]()
This reaction being reversible, CO2 is removed as soon as it is produced to enable the reaction to proceed to completion.
![]()
Question 32.
What is slaking of lime?
Answer:
The addition of limited amount of water breaks the lump of lime. This process is called slaking
of lime and the product is slaked lime.
CaO + H2O → Ca(OH)2
Question 33.
What happens when quick lime reacts with –
1. H2O
2. CO2?
Answer:
1. CaO + H2O → Ca(OH)2 (calcium hydroxide)
2. CaO + CO2 → CaCO3 (calcium carbonate)
Question 34.
Prove that calcium oxide is a basic oxide.
Answer:
Calcium oxide is a basic oxide. It combines with acidic oxides at high temperature.
Question 35.
Mention the uses of quick lime.
Answer:
Calcium oxide (quick lime) is used
- to manufacture cement, mortar and glass.
- in the manufacture of sodium carbonate and slaked lime.
- in the purification of sugar.
- as drying agent.
Question 36.
What is milk of lime? How CO2 reacts with it?
Answer:
The aqueous solution of calcium hydroxide is known as lime water and a suspension of slaked lime in water is known as milk of lime. When carbon dioxide is passed through lime water, it turns milky due to the formation of calcium carbonate.
Ca(OH)2 + CO2 → CaCO2 + H2O
Question 37.
What happens when excess of CO2 reacts with calcium carbonate?
Answer:
CaCO3 + CO2 + H2O → Ca(HCO3)2 (Calcium bi-carbonate)
Question 38.
What is bleaching powder? How Is it prepared?
Answer:
Bleaching powder is Ca(OCl)2. it is prepared by treating chlorine with milk of lime.
![]()
Question 39.
What are the uses of calcium hydroxide?
Answer:
Calcium hydroxide is used
- in the preparation of mortar, a building material.
- in white wash due to its disinfectant nature.
- in glass making and tanning industry.
- for the preparation of bleaching powder and for the purification of sugar.
Question 40.
How gypsum occurs in nature?
Answer:
- Gypsum is CaSO4.2H2O. Gypsum beds were formed due to the evaporation of water from the massive prehistoric sea basins.
- When water evaporates, the minerals present in it become concentrated and crystallized.
- Gypsum is formed, due to evaporation, sulphur present in water bonds with oxygen to form a sulphate. The sulphate then combines with calcium and water to form gypsum.
Question 41.
How is gypsum synthesized?
Answer:
Gypsum can also be synthesized from coal-fired power plants, as a by-product of flue-gas de suiflirization. The process of scrubbing sulfur from flue gases produced when coal is burned results in the production of several by-products, including gypsum.
Question 42.
What is meant by retrograde solubility?
Answer:
Gypsum is a soft mineral and it is less soluble in water as the temperature increases. This is known as retrograde solubility, which is a distinguishing characteristic of gypsum.
![]()
Question 43.
Write a note about physical appearance of gypsum.
Answer:
- Gypsum is usually white, colorless or grey in colour.
- It can also be found in the shades of pink, yellow, brown and light green, mainly due to the presence of impurities.
- Gypsum crystals are found to occur in a form that resembles the petals of a flower. This type of formation is referred to as ‘desert rose’, as they mostly occur in arid areas or desert terrains.
Question 44.
Prove that gypsum is a natural insulator.
Answer:
- Gypsum have low thermal conductivity.
- It won’t allow the electric current to pass through it. So it is known as natural insulator.
Question 45.
Write a note about alabaster.
Answer:
- Alabaster is a variety of gypsum.
- It is highly valued as an ornamental stone.
- it has been used by the sculptors for centuries.
- Alabaster is granular and opaque.
Question 46.
What ¡s dead burnt plaster?
Answer:
When Plaster of Paris is heated above 393K, no water of crystallisation is left and anhydrous calcium sulphate (CaSO4) is formed. This is known as ‘dead burnt plaster’.
Question 47.
What ¡s meant by the setting of cement?
Answer:
When gypsum is added to cement by mixing with an adequate quantity of water, it forms a plastic mass that gets into a hard solid in 5 to 10 minutes.
Question 48.
Write notes on plaster of paris.
Answer:
It is a hemihydrate of calcium sulphate. It is obtained when gypsum, CaSO4.2H2O, is heated to 393 K.
2CaSO4 2H2O{s) → 2CaSO4 .H2)O + 3H2O
Above 393 K, no water of crystallisation is left and anhydrous calcium sulphate, CaSO4 is formed. This is known as ‘dead burnt plaster’. It has a remarkable property of setting with water. On mixing with an adequate quantity of water it forms a plastic mass that gets into a hard solid in 5 to 15 minutes.
Uses:
The largest use of Plaster of Paris is in the building industry as well as plasters. It is used for immobilizing the affected part of organ where there is a bone fracture or sprain. It is also employed in dentistry, in ornamental work and for making casts of statues and busts.
Question 49.
In what ways lithium shows similarities to magnesium in its chemical behaviour?
Answer:
- Both react with nitrogen to form nitrides.
- Both react with O2 to form monoxides.
- Both the elements have the tendency to form covalent compounds.
- Both can form complex compounds.
Question 50.
Explain why can alkali and alkaline earth metals not be obtained by the chemical reduction method.
Answer:
Alkali and alkaline earth metals, themselves acts as better recurring agents and reducing agents, better than alkali metals. That is why these metals are not obtained by chemical reduction methods.
Question 51.
Why are potassium and caesium. rather than lithium used ¡n photoelectric cells?
Answer:
Potassium and caesium have much lower ionization enthalpy than that of lithium. As a result, these metals easily emit electrons on exposure to light. Due to this, K and Cs are used in photoelectric cells rather than lithium.
![]()
Question 52.
Berllium and magnesium do not give colour to flame whereas other alkaline earth metals do so. Why?
Answer:
Due to small size, the ionization enthalpies of Be and Mg are much higher than those of other alkaline earth metals. Therefore, a large amount of energy is needed to excite their valence electron and that’s why they do not impart colour to the flame.
Question 53.
Why are lithium salts commonly hydrated and those of the other alkali metal ions usually anhydrous?
Answer:
Due to its smallest size, Li+ can polarize water molecules easily than the other alkali metal ions.
Question 54.
Why are alkali metals always univalent? Which alkali metal ion forms largest hydrated ion in aqueous solution?
Answer:
They are always univalent because after losing one electron, they acquire nearest inert gas configuration. Li+ forms largest hydrated cations because it has the highest hydration energy.
Question 55.
What is the effect of heat on the following compounds (Give equations for the reactions)?
Answer:
1. CaCO3
2. CaSO4 .2H2O
Answer:

Question 56.
Explain the following:
Answer:
(a) Lithium Iodide is more covalent than lithium fluoride.
(b) Lattice enthalpy of LIF is maximuni among all the alkali metal halides.
Answer:
(a) According to Fazan’s rule, Li+ ion can polarise I– ion more than the F– ion due to bigger size of the anion. Thus Li+ has more covalent character than LiF.
(b) Smaller the size (internuclear distance), more is the value of lattice enthalpy since internuclear distance is expected to be least in the LiF.
Question 57.
Why alkali metals are soft and have low melting points?
Answer:
Alkali metals have only one valence electron per metal atom. As a result, the binding energy of alkali metal ions in the close-packed metal lattices are weak. Therefore, these are soft and have low melting point.
![]()
Question 58.
Why is LiF almost insoluble In water whereas LiCl soluble not only in water but also in acetone?
Answer:
The low solubiLity of LiF in water is due to its very high Lattice enthalpy (F– ion is very small in size). On the other hand, in lithium chloride (LiCl) the lattice enthaipy is comparatively very small. This means that the magnitude of hydration enthalpy is quite large. Therefore lithium chloride dissolves in water. It is also soluble in acetone due to dipolar attraction (Acetone is polar in nature).
Question 59.
The hydroxides and carbonates of sodium and potassium are easily soluble in water while the corresponding salts of magnesium and calcium are sparingly soluble in water. Explain.
Answer:
Since group 1 hydroxides and carbonates due to large size contain higher hydration energy than the lattice energy so, they are easily soluble in water. Where as, in magnesium and calcium due to small size their lattice energy dominates over hydration energy they are sparingly soluble in water.
Question 60.
Draw the tile structure of –
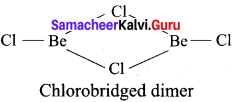
1. BeCl2 (vapour)
2. BeCl2 (solid).
Answer:
BeCl2 (vapour):
In the vapour state, it exists as a chlorobridged dimer.
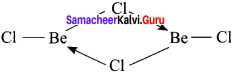
2. BeCl2 (solid):
Question 61.
Why is Li2CO3 decomposed at a lower temperature whereas Na2CO3 at higher temperature?
Answer:
Li2CO3 is a covalent compound, whereas Na2CO3 is an ionic compound. Therefore, lattice energy of Na2CO3 is higher than that of Li2CO3. Thus, LiCO3 is decomposed at a lower temperature.
Question 62.
Alkali metals give colouration when heated in Bunsen flame. Give reason.
Answer:
1. When the alkali metals salts moistened with concentrated hydrochloric acid are heated on a platinum wire in a flame, they show characteristic coloured flame.
- Lithium – Crimson red
- Sodium – Yellow
- Potassium – Lilac
- Rubidium – Reddish violet
- Caesium – Blue
2. The heat in the flame excites the valence electron to a higher energy level. When it drops back to its actual energy level, the excess energy is emitted as light whose wavelength is in the visible region produces colour.
![]()
Question 63.
How sodium metal reacts with –
1. ethanol
2. acetylene.
Answer:
(i) Sodium metal reacts with ethanol to form sodium ethoxide and liberates H2 gas.
![]()
![]()
Question 64.
Mention the uses of lithium and its compounds. .
Answer:
- Lithium metal is used to make useful alloys. For e.g. with lead, lithium is used to make white metal bearings for motor engines, with aluminium to make aircraft parts and with magnesium to make armour plates. It is also used in thermonuclear reactions.
- Lithium is used to make electrochemical cells.
- Lithium carbonate is used in medicines.
Question 65.
What are the uses of sodium and its compounds?
Answer:
- Sodium is used to make Na/Pb alloy needed to make Pb(Et)4 and Pb(Me)4. These organolead compounds were used as anti-knock additives to petrol in early days.
- Liquid sodium metal is used as a coolant in fast breeder nuclear reactors.
Question 66.
What are the uses of potassiunt and its compounds?
Answer:
- Potassium has a vital role in biological system.
- Potassium chloride is used as a fertilizer.
- Potassium hydroxide is used in the manufacture of soft soap.
- Potassium hydroxide is also used as an excellent absorbent of carbon dioxide.
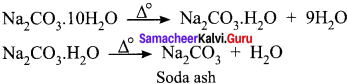
Question 67.
What is soda ash? How is it obtained?
Answer:
Sodium carbonate decahydrate commonly known as washing soda Na2CO3. 10H2O. Upon heating, it looses the water of crystallization to form monohydrate. Above 373K, the monohydrate becomes completely anhydrous and changes to a white powder called soda ash.
Question 68.
Mention the uses of washing soda (or) sodium carbonate.
Answer:
- Sodium carbonate is highly used in laundering.
- It is an important laboratory reagent used in qualitative analysis and in volumetric analysis.
- It is also used in water treatment to convert hard water to soft water.
- It is used in the manufacture of glass, paper, and paint.
Question 69.
How would you prepare pure sodium chloride from crude salt?
Answer:
(a) Crude salt contains sodium sulphate, calcium sulphate, calcium chloride and magnesium chloride as impurities along with sodium chloride.
(b) Pure NaCl is obtained from crude salt by removal of insoluble impurities through filtration from the crude salt solution with a minimum amount of water.
(c) Sodium chloride can be crystallized by passing HCl gas into this solution.
(d) Calcium and magnesium chloride being more soluble than NaCl, remain in the solution.
![]()
Question 70.
Mention the uses of sodium chloride.
Answer:
- It is used as a common salt (or) table salt for domestic purposes.
- It is used for the preparation of many inorganic compounds such as NaOH and Na2CO3.
Question 71.
What are the uses of sodium hydroxides?
Answer:
- Sodium hydroxide is used as a laboratory reagent.
- It is used in the purification of bauxite and petroleum refining.
- It is used in the textile industries for mercerizing cotton fabrics.
- It is used in the manufacture of soap, paper, artificial silk, and a number of chemicals.
Question 72.
Give a reason why sodium bicarbonate is used in bakeries.
Answer:
Sodium bicarbonate is called baking soda. Because it decomposes on heating to generate bubbles of carbon dioxide, leaving holes in cakes or pastries and making them light and fluffy.
Question 73.
Write about the uses of sodium bicarbonate.
Answer:
- NaHCO3 is used as an ingredient in baking.
- It is used as a mild antiseptic for skin infections.
- It is also used in fire extinguishers.
Question 74.
Explain the action of soda-lime with
1. SiO3
2. P4O10.
Answer:
Quick lime mixed with soda gives solid soda lime. It combines with acidic oxides such as SiO2, ançi P4O10 to form calcium silicate and calcium phosphate, respectively.

Samacheer Kalvi 11th Chemistry Alkali and Alkaline Earth Metal 3-Mark Questions
Question 1.
Explain the periodic nature of ionization enthalpy in the alkali group.
Answer:
- Alkali metals have the lowest ionization enthalpy in each period.
- Within the group, as we go down, the ionization enthalpies of alkali metals decrease due to the increase in atomic size.
- In large atoms, the valence electrons are loosely held by the nucleus and arc easily lost, leading them to have low ionization enthalpy and acquiring stable noble gas configuration.
- On moving down the group, the atomic size increases and the number of inner shells also increases, which in turn increases the magnitude of screening effect. So. the ionization enthalpies decreases down the group.
Question 2.
Discuss the reaction of alkali metals with liquid ammonia.
Answer:
Alkali metals dissolve in liquid ammonia to give deep blue solutions that are conducting in nature. The conductivity is similar to that of pure metals. This happens because the alkali metal atom readily loses its valence electron in ammonia solution. Both the cation and the electron are ammoniated to give ammoniated cation and ammoniated electron.
M + (x + y)NH3 → [M(NH3)x]+ [e(NH3)y)]–
The blue colour of the solution is due to the ammoniated electron which absorbs energy in the visible region of light and thus imparts blue colour to the solution. The solutions are paramagnetic and on standing slowly liberate hydrogen resulting in the formation of an amide.
M+ + e– + NH3 → MNH2 + \(\frac{1}{2}\)H2
In concentrated solution, the blue colour changes to bronze colour and become diamagnetic.
Question 3.
Explain the anomalous behaviour of lithium among the alkali metals.
Answer:
- Lithium is extremely small.
- It has great polarizing power.
- It has least electropositive character.
- In lithium, the non-availability of d-orbitals is observed.
Question 4.
Write the uses of sodium carbonate.
Answer:
- Sodium carbonate known as washing soda is used heavily for laundering
- It is an important laboratory reagent used in qualitative analysis and in volumetric analysis.
- It is also used in water treatment to convert hard water to soft water
- It is used in the manufacturing of glass, paper, paint, etc…
![]()
Question 5.
How alkali metals react with liquid ammonia?
Answer:
- Alkali metals dissolve in liquid ammonia to give deep blue solutions that are conducting in nature.
- This happens because the alkali metal atom readily loses the valence electron in ammonia solution.
- Both the cation and the electron combine with ammonia to form ammoniated cation and ammoniated electron. M + (x+v)NH3 → [M(NH3)x]+ + [e(NH3)y]–
Question 6.
What is the reason behind the blue colouration of alkali metals with liquid ammonia?
Answer:
M + (x + v)NH3 → [M(NH3)x]+ + [e(NH3)y]–
The blue colour of the solution is due to the ammoniated electron which absorbs energy in the visible region of light and thus imparts blue colour to the solution. The solutions are paramagnetic and on standing slowly liberate hydrogen resulting in the formation of amide. In concentrated solution, the blue colour changes to bronze colour and become diamagnetic.
Question 7.
Compare the ionization energy of alkali metals with alkaline earth metals.
Answer:
Members of group 2 have higher ionization enthalpy values than group 1 because of their smaller size, with electrons being more attracted towards the nucleus of the atoms. Correspondingly they are less electropositive than alkali metals.
Although IE1 values of alkaline earth metals are higher than that of alkali metals, the IE2 values of alkaline earth metals are much smaller than those of alkali metals. This occurs because in alkali metals the second electron is to be removed from a cation, which has already acquired a noble gas configuration.
In the case of alkaline earth metals, the second electron is to be removed from a monovalent cation, which still has one electron in the outermost shell. Thus, the second electron can be removed more easily in the case of group 2 elements than in group 1 elements.
Question 8.
Describe the fireworks of alkaline earth metals.
Answer:
- Combined with the element of chlorine, barium sends up a green spark.
- Strontium chloride flashes red.
- Copper and chlorine compound makes a blue firework.
- Magnalium – A mixture of the alkaline earth metal magnesium and aluminium boosts all fireworks colours, particularly makes the blue brighter.
Question 9.
Write the uses of calcium.
Answer:
- As a reducing agent in the metallurgy of uranium, zirconium and thorium.
- As a deoxidiser, desulphuriser or decarboniser for various ferrous and non-ferrous alloys.
- In making cement and mortar to be used in construction.
- As a getter in vacuum tubes.
- In dehydrating oils
- In fertilisers, concrete and plaster of paris.
Question 10.
IE1of alkaline earth metals are higher than that of alkali metals, but IE2 of alkaline earth metals are smaller than that of alkali metals. Give reason.
Answer:
- IE1 of alkaline earth metals > IE1 of alkali metals.
- IE2 of alkaline earth metals < IE2 of alkali metals.
- This occurs because in alkali metals the second electron is to be removed from a cation, which has already acquired a noble gas configuration.
- In the case of alkaline earth metals, the second electron is to be removed from a monovalent cation, which still has one electron in the outermost shell.
- Thus, the second electron can be removed more easily in the case of group 2 elements than in group I elements.
Question 11.
MgCl2 and CaCl2 are easily hydrated, while NaCl and KCl are not hydrated. Why?
Answer:
Compounds of alkaline earth metals are more extensively hydrated than those of alkali metals, because the hydration enthalpies of alkaline earth metal ions are larger than those of alkali metal ions.
e.g., MgCl2 and CaCl2 exist as MgCl2.6H2O and CaCl2.6H2O, while NaCl and KCl do not form such hydrates.
![]()
Question 12.
What is the distinctive behaviour of beryllium?
Answer:
- Beryllium is small in size.
- It has high polarizing power.
- Its electronegativity is relatively high.
- It has high ionization enthalpy.
- In valence shell, vacant d-orbitais are absent in beryllium.
Question 13.
Write about the important uses of calcium. Calcium is used
Answer:
- As a reducing agent in the metallurgy of uranium, zirconium and thorium.
- As a de oxidizer, desulfurizer or decabonizer for various ferrous and non-ferrous alloys.
- in making of cements and mortars to be used in construction.
- As a getter in vacuum tubes.
- In dehydrating oils
- In fertilizers, concrete and Plaster of Paris.
Question 14.
Mention about the uses of barium. Barium is used
Answer:
- In metallurgy, its compounds are used in pyrotechnics, petroleum mining, and radiology.
- De oxidizer in copper refining.
- Its alloys with nickel readily emits electrons hence used in electron tubes and in spark plug electrodes.
- As a scavenger to remove last traces of oxygen and other gases in television and other electronic tubes.
- An isotope of barium ‘33Ba., used as a source in the calibration of gamma-ray detectors in nuclear chemistry.
Question 15.
Be(OH)2 is amphoteric in nature. Prove it.
Answer:
Be(OH)2 is amphoteric in nature as it reacts with both acid and alkali.

Question 16.
Write a note about the structure of beryllium chloride.
Answer:
- BeCl2 has a chain structure in the solid-state.
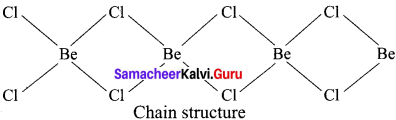
- In the vapour phase BeCl2 tends to form a chloro-bridged dimer.
- At high temperatures of the order of 1200K it gives linear monomer.
Question 17.
Draw the structure of BeCl2 in different physical states.
Answer:
1. In solid-state:

2. in vapour state:
3. in High temperature:
![]()
Question 18.
Write about the suiphates of alkaline earth metals.
Answer:
- The sulphates of the alkaline earth metals are all white solids and stable to heat.
- BeSO4 and MgSO4 are readily soluble in water; the solubility decreases from CaSO4 to BaSO4.
- The greater hydration enthalpies of Be2and Mg2 ions overcome the lattice enthalpy factor and therefore their suiphates are soluble in water.
Solubility order : BeSO4 < CaSO4 < BaSO4
Question 19.
What are the common physical and chemical features of alkali metals?
Answer:
Physical properties of alkali metals :
- Alkali metals have low ionization enthalpies.
- Alkali metals are highly electropositive in nature.
- Alkali metals exhibit +1 oxidation states in their compounds.
- Alkali metals impart characteristic colours to the flame.
Chemical properties of alkali metals:
- Alkali metals are highly reactive in nature.
- Alkali metals hydroxides are highly basic in nature.
- Alkali metals dissolve in liquid ammonia to form blue and conducting solution.
Question 20.
Compare the alkali metals and alkaline earth metals with respect to –
- ionization enthalpy
- basicity of oxides and
- solubility of hydroxides.
Answer:
1. Ionization enthalpy:
Because of high nuclear charge the ionization enthalpy of alkaline earth metals are higher than those of the corresponding alkali metals.
2. Basicity of oxides:
Basicity of oxides of alkali metals are higher than that of alkaline earth metals.
3. Solubility of hydroxides:
Solubility of hydroxides of alkali metals are higher than that of alkaline earth metals. Alkali metals due to lower ionization enthalpy are more electropositive than the corresponding group 2 elements.
![]()
Question 21.
Why is Li2CO3 decomposed at a lower temperature, whereas Na2CO3 at a higher temperature?
Answer:
Li2CO3 is a covalent compound, whereas Na4CO3 is an ionic compound. Therefore, the lattice energy of Na2CO3 is higher than that of Li2CO4. Thus, Li2CO3 is decomposed at a lower temperature.
Question 22.
What happens when
- Sodium metal is dropped in water?
- Sodium metal is heated in free supply of air?
- Does sodium peroxide dissolve in water?
Answer:
- 2Na + 2H2O —‘ 2NaOH + H2
- 2Na + O2 —+ Na2O2
- Na2O2+ 2H2O —‘ 2NaOH + H202
Question 23.
Write balanced equations for reactions between
- Na2O2 and water
- KO2and water
- Na2O and CO2
Answer:
- Na2O2 + 2H2O → 2NaOH + H2O2
- 2KO2 + 2H2O → 2KOH + O2 + H2O2
- Na2O + CO2 → Na2CO3
Question 24.
How would you explain the following observations?
- BeO is almost insoluble but BeSO4 is soluble ¡n water.
- BaO is soluble but BaSO4 is insõluble in water.
- LiI is more soluble than KI in ethanol.
Answer:
- Lattice energy of BeO is comparatively higher than the hydration energy. Therefore, it is almost insoluble in water. Whereas BeSO4 is ionic in nature and its hydration energy dominates the lattice energy.
- Both BaO and BaSO4 are ionic compounds but the hydration energy of BaO is higher than the lattice energy, therefore it is soluble in water.
- Since the size of Li+ ion is very small in comparison to K+ ion, it polarises the electronb cloud of I– ion to a great extent. Thus LiI dissolves in ethanol more easily than the KI.
Question 25.
Explain the following:
- Why Cs is considered as the most electropositive element?
- Lithium cannot be used in making photoelectric cells.
- Lithium does not form alums.
Answer:
- Due to its lowest ionization energy, Cs is considered as the most electropositive element.
- Lithium cannot be used in making photoelectric cells because out of all the alkali metals, it has highest ionization energy and thus cannot emit electrons when exposed to light.
- Due to small size, lithium does not form alums.
Question 26.
Give the important uses of the following compounds.
1. NaHCO3
2. NaOH
Answer:
1. Uses of NaHCO3
- It is used in fire extinguisher.
- It is used as mild antiseptic for skin infections.
- It is used as antacid.
2. Uses of NaOH
- It is used in soap industry
- It is used as reagent in laboratory
- It is used in absorbing poisonous gases.
Question 27.
The hydroxides and carbonates of sodium and potassium are easily soluble in water, while the corresponding salts of magnesium and calcium are sparingly soluble in water. Explain.
Answer:
All the compounds are crystalline solids and their solubility in water is guided by both lattice enthalpy and hydration enthalpy. The magnitude of lattice enthalpy is quite small in case of sodium and potassium compounds, hence they are readily dissolved in water, when compared to magnesium and calcium compounds.
However, in case of corresponding magnesium and calcium compounds, the cations have smaller sizes and more magnitude of positive charge. This means that their lattice enthalpies are more, when compared to the sodium and potassium compounds. Therefore, the hydroxi des and carbonates of these metals are only sparingly soluble in water.
![]()
Question 28.
Why is LiF almost insoluble in water, whereas LiCl soluble not only in water but also In acetone?
Answer:
The low solubility of LiF in water is due to its very high lattice enthalpy (F– ion is very small in size). On the other hand, in lithium chloride (LiCl) the lattice enthalpy is comparatively very small. This means that the magnitude of hydration enthalpy is quite large. Therefore lithium chloride dissolves in water. It is also soluble in acetone due to dipolar attraction (Acetone is polar in nature).
Question 29.
Which out of the following can be used to store an alkali metal?
1. H2O
2. C2H5 OH
3. benzene
Answer:
3. Benzene can be used to store an alkali metal, because other substances react with alkali metal as below:
Na + H2O → NaOH + ½H2
Na + C2H5COH → C2H5ONa + ½H2
Samacheer Kalvi 11th Chemistry Alkali and Alkaline Earth Metal 5 – Mark Questions
Question 1.
Explain in what respects lithium ¡s different from other metals of the same group.
Answer:
lithium:
- Very hard.
- High melting and boiling point.
- Least reactive.
- Reacts with nitrogen to get Li3N.
- Reacts with bromine slowly.
- Burnt in air gives monoxide only.
- Compounds are partially soluble in water.
- Lithium nitrate decomposes to fòrm an oxide.
- Extremely small in size.
- Li+ has greater polarizing power.
Other elements of the family:
- Very Soft.
Low melting and boiling point. - More reactive.
- No reaction.
- Reacts violently.
- Burnt in air gives peroxides also, apart from monoxides. K, Rb and Cs gave super oxides.
- Highly soluble in water.
- Other metals on heating gives nitrite.
- Comparatively large in size.
- Other M+ ions have comparatively larger polarizing power.
Question 2.
Discuss the diagonal relationship between Lithium and Magnesium.
Answer:
Similarity between the first member of group 1 (Li) and the diagonally placed second clement of group 2 (Mg) is called diagonal relationship. It is due to similar size (rLi+ = 0.766 Å and Mg2+ = 0.72 Å) and comparable electronegativity values (Li = 1.0; Mg = 1.2). Similarities between Lithium and Magnesium are
- Both lithium and magnesium are harder than other elements in the respective groups
- Lithium and magnesium react slowly with water. Their oxides and hydroxides are much less soluble and their hydroxides decompose on heating.
- Both form a nitride, Li3N, and Mg3N2, by direct combination with nitrogen
- They do not give any superoxides and form only oxides, Li2O and MgO
- The carbonates of lithium and magnesium decompose upon heating to form their respective oxides and CO2.
- Lithium and magnesium do not form bicarbonates.
- Both LiCl and MgCl2 are soluble in ethanol and are deliquescent. They crystallize from aqueous solution as hydrates, LiCl.2H2O and MgCl28H2O
Question 3.
Compare the properties of beryllium with the other elements in the same group.
Answer:
Beryllium:
- Forms covalent compounds.
- High melting and boiling point.
- Does not react with water even at elevated temperature.
- Does not combine directly with hydrogen.
- Halides are covalent.
- Hydroxides and oxides of beryllium are amphoteric in nature.
- It is not readily attacked by acids because of the presence of an oxide film.
- Beryllium carbide evolves methane withwater.
- Salts of Be are extensively hydrolyzed.
- It has no vacant ‘d’ orbitals in the outermost shell.
Other elements of the family:
- Forms ionic compounds.
- Lower melting and boiling point.
- React with water.
- Combine directly with hydrogen.
- Halides are ionic or electrovalent.
- Hydroxides and oxides are basic in nature.
- Readily attacked by acids.
- Other carbides evolves acetylene with water.
- Hydrolyzed.
- They have vacant ‘d’ orbitais in the outermost shell.
Question 4.
Write the uses of alkali metals.
Answer:
- Lithium metal is used to make useful alloys. For example, lead is used to make ‘white metal’ bearings for motor engines, with aluminium to make aircraft parts, and with magnesium to make armour plates. It is used in thermonuclear reactions.
- Lithium is also used to make electrochemical cells.
- Lithium carbonate is used in medicines
- Sodium is used to make Na/Pb alloy needed to make Pb(Et)4 and Pb(Me)4. These organolead compounds were earlier used as anti-knock additives to petrol, but nowadays lead-free petrol in use.
- Liquid sodium metal is used as a coolant in fast breeder nuclear reactors. Potassium has a vital role in biological systems.
- Potassium chloride is used as a fertilizer. Potassium hydroxide is used in the manufacture of soft soap. It is also used as an excellent absorbent of carbon dioxide.
- Caesium is used in devising photoelectric cells.
Question 5.
Distinguish between alkali metals and alkaline earth metals.
Answer:
Alkali Metals:
- Alkali metals are soft.
- They have a single electron in the valence shell and their electronic configuration is [noble gas] ns1.
- They have low melting points.
- Hydroxides are strongly basic.
- Carbonates do not decompose.
- Carbonates do not decompose.
- Nitrates give corresponding nitrites and oxygen as products.
- They show +1 oxidation states.
- Their carbonates are soluble in water except for Li2CO3.
- Except for Li, alkali metals do not form complex compounds.
Alkaline earth metals:
- Alkaline earth metals are hard.
- They have two electrons in the valence shell and their electronic configuration is [noble gas] ns2.
- They have relatively high melting points.
- Hydroxides are less basic.
- Carbonates decompose to form oxide when heated to high temperatures.
- Carbonates decompose to form oxide when heated to high temperatures.
- Nitrates give corresponding oxides, nitrogen dioxide, and oxygen as products.
- They show +2 oxidation states.
- Their carbonates are insoluble in water.
- They can form complex compounds.
Question 6.
Explain the diagonal relationship of Beryllium with Aluminium.
Answer:
Beryllium (the first member of group 2) shows a diagonal relationship with aluminium. In this case, the size of these ions (rBe2+ = 0.45 Å and rAl3+ = 0.54 Å) is not as close. However, their charge per unit area is closer. (Be2+ = 2.36 and Al3+ = 2.50) They also have same electronegativity values (Be = 1.5; Al = 1.5).
Properties:
- Beryllium chloride forms a dimeric structure like aluminium chloride with chloride bridges. Beryllium chloride also forms polymeric chain structure in addition to dimer. Both are soluble in organic solvents and are strong Lewis acids.
- Beryllium hydroxide dissolves in excess of alkali and gives beryllate ion and [Be(OH)4]2- and hydrogen as aluminium hydroxide which gives aluminate ion. [Al( OH4)–
- Beryllium and aluminum ions have strong tendency to form complexes, BeF42-, AlF63-.
- Both beryllium and aluminium hydroxides are amphoteric in nature.
- Carbides of beryllium (Be2C) like aluminum carbide (Al4C3) give methane on hydrolysis
- Both beryllium and aluminium are rendered passive by nitric acid.
![]()
Question 7.
An alkali metal (A) belongs to period number II and group number I react with oxygen to form (B). (A) reacts with water to form (C) with the liberation of hydrogen compound (D). Identify A, B, C, and D.
Answer:
1. An alkali metal (A) belongs to period number II and group number I is lithium.
2. Lithium reacts with oxygen to form simple oxide lithium oxide (B).
![]()
3. Lithium reacts with water to form lithium hydroxide with the liberation of hydrogen.

4. Lithium directly react with carbon to form an ionic compound lithium carbide.
Question 8.
Describe the Solvay process (or) how is washing soda (or) sodium carbonate prepared in industries?
Answer:
(i) Solvay process – in this process ammonia is converted to ammonium carbonate, which is then converted to ammonium bicarbonate bypassing excess carbon dioxide in sodium chloride solution saturated with ammonia.
(ii) The ammonium bicarbonate formed reacts with sodium chloride to give sodium bicarbonate.
As sodium bicarbonate has poor solubility, it gets precipitated.
(iii) The sodium bicarbonate is isolated and is heated to give sodium carbonate.
(iv) The equations involved in this process is as below:
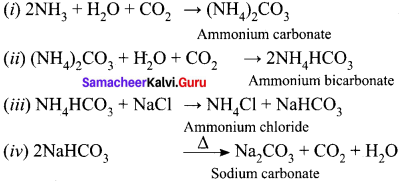
Question 9.
Describe the method of electrolysis of brine solution? (or) how is sodium hydroxide prepared commercially?
Answer:
(a) Sodium hydroxide is prepared commercially by the electrolysis of brine solution in Castner-Kellner cell using a mercury cathode and a carbon anode.
(b) Sodium metal is discharged at the cathode and combines with mercury to form sodium amalgam.
(c) Chlorine gas is evolved at the cathode.
(d) The sodium amalgam thus obtained is treated with water to give sodium hydroxide.