Students can Download Bio Botany Chapter 8 Biomolecules Questions and Answers, Notes Pdf, Samacheer Kalvi 11th Bio Botany Book Solutions Guide Pdf helps you to revise the complete Tamilnadu State Board New Syllabus and score more marks in your examinations.
Tamilnadu Samacheer Kalvi 11th Bio Botany Solutions Chapter 8 Biomolecules
Samacheer Kalvi 11th Bio Botany Biomolecules Text Book Back Questions and Answers
I. Multiple Choice Questions
Choose the correct answer
Question 1.
The most basic amino acid is …………… .
(a) Arginine
(b) Histidine
(c) Glycine
(d) Glutamine
Answer:
(a) Arginine
Question 2.
An example of feedback inhibition is …………… .
(a) Cyanide action on cytochrome
(b) Sulpha drug on folic acid synthesiser bacteria
(c) Allosteric inhibition of hexokinase by glucose – 6 – phosphate
(d) The inhibition of succinic dehydrogenase by malonate
Answer:
(c) Allosteric inhibition of hexokinase by glucose – 6 – phosphate
![]()
Question 3.
Enzymes that catalyse interconversion of optical, geometrical or positional isomers are …………… .
(a) Ligases
(b) Lyases
(c) Hydrolases
(d) Isomerases
Answer:
(d) Isomerases
Question 4.
Proteins perform many physiological functions. For example some functions as enzymes. One of the following represents an additional function that some proteins discharge …………… .
(a) Antibiotics
(b) Pigment conferring colour to skin
(c) Pigments making colours of flowers
(d) Hormones
Answer:
(d) Hormones
Question 5.
Given below is the diagrammatic representation of one of the categories of small molecular weight organic compounds in the living tissues. Identify the category shown & one blank component “X” in it …………… .
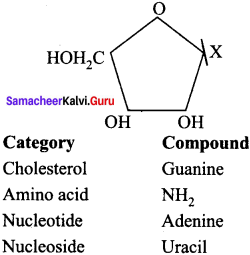
Answer:
(a) Nucleoside
(b) Uracil.
![]()
Question 6.
Distinguish between nitrogenous base and a base found in inorganic chemistry.
Answer:
Nitrogenous Base:
- Nitrogenous bases are organic molecules containing the element nitrogen & acts as a base in chemical reaction.
- e.g. Adenine, Thymine
Base:
- Bases are the substance that release hydroxide (OH– ) ions in aqueous solution.
- e.g. NaOH and Ca(OH)2
Question 7.
What are the factors affecting the rate of enzyme reaction?
Answer:
(a) Temperature: Heating increases molecular motion. Thus the molecules of the substrate and enzyme move more quickly resulting in a greater probability of occurrence of the reaction. The temperature that promotes maximum activity is referred to as optimum temperature.
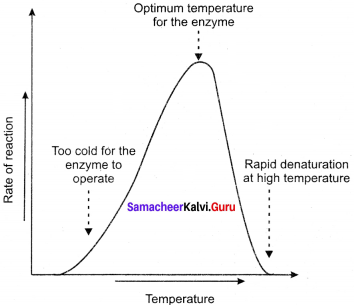
(b) pH: The optimum pH is that at which the maximum rate of reaction occurs. Thus the pH change leads to an alteration of enzyme shape, including the active site. If extremes of pH are encountered by an enzyme, then it will be denatured.
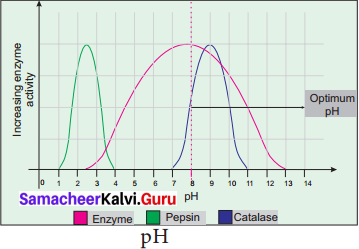
(c) Substrate Concentration: For a given enzyme concentration, the rate of an enzyme reaction increases with increasing substrate concentration.
(d) Enzyme Concentration: The rate of reaction is directly proportional to the enzyme concentration.
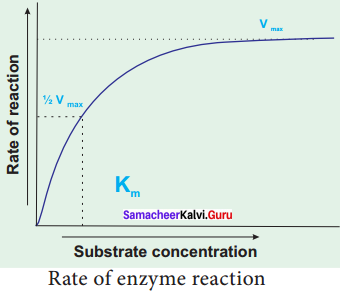
The Michaelis – Menton Constant (Km) and Its Significance:
When the initial rate of reaction of an enzyme is measured over a range of substrate concentrations (with a fixed amount of enzyme) and the results plotted on a graph. With increasing substrate concentration, the velocity increases – rapidly at lower substrate concentration. However the rate increases progressively, above a certain concentration of the substrate the curve flattened out. No further increase in rate occurs. This shows that the enzyme is working at maximum velocity at this point. On the graph, this point of maximum velocity is shown as VMax.
![]()
Question 8.
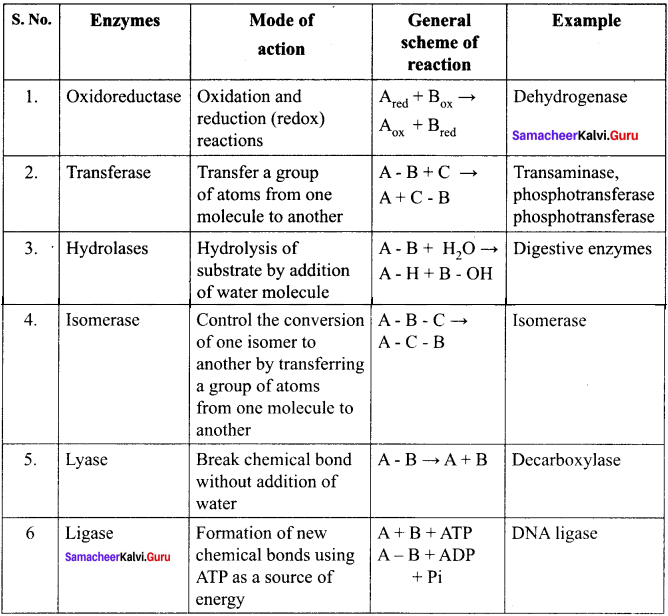
Briefly outline the classification of enzymes.
Answer:
Enzymes are classified into six groups based on their mode of action.
Question 9.
Write the characteristic feature of DNA.
Answer:
The characteristic feature of DNA.
- If one strand runs in the 5′ – 3′ direction, the other runs in 3′ – 5′ direction and thus are antiparallel (they run in opposite direction). The 5′ end has the phosphate group and 3’end has the OH group.
- The angle at which the two sugars protrude from the base pairs is about 120°, for the narrow angle and 240° for the wide angle. The narrow angle between the sugars generates a minor groove and the large angle on the other edge generates major groove.
- Each base is 0.34 nm apart and a complete turn of the helix comprises 3.4 nm or 10 base pairs per turn in the predominant B form of DNA.
- DNA helical structure has a diameter of 20 Å and a pitch of about 3 Å. X – ray crystal study of DNA takes a stack of about 10 bp to go completely around the helix (360°).
- Thermodynamic stability of the helix and specificity of base pairing includes
- (a) The hydrogen bonds between the complementary bases of the double helix
- (b) stacking interaction between bases tend to stack about each other perpendicular to the direction of helical axis. Electron cloud interactions (\({ \Pi -{ \Pi } }\)) between the bases in the helical stacks contribute to the stability of the double helix.
- The phosphodiester linkages gives an inherent polarity to the DNA helix. They form strong covalent bonds, gives the strength and stability to the polynucleotide chain.
- Plectonemic coiling – the two strands of the DNA are wrapped around each other in a helix, making it impossible to simply move them apart without breaking the entire structure. Whereas in paranemic coiling the two strands simply lie alongside one another, making them easier to pull apart.
- Based on the helix and the distance between each turns, the DNA is of three forms – A DNA, B DNA and Z DNA.
Question 10.
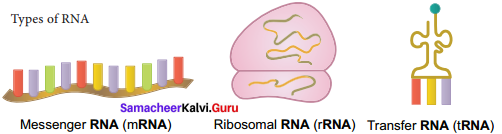
Explain the structure and function of different types of RNA.
Answer:
1. mRNA (messenger RNA): Single stranded, carries a copy of instructions for assembling amino acids into proteins. It is very unstable and comprises 5% of total RNA polymer. Prokaryotic mRNA (Polycistronic) carry coding sequences for many polypeptides. Eukaryotic mRNA (Monocistronic) contains information for only one polypeptide.
2. tRNA (transfer RNA): Translates the code from mRNA and transfers amino acids to the ribosome to build proteins. It is highly folded into an elaborate 3D structure and comprises about 15% of total RNA. It is also called as soluble RNA.
3. rRNA (ribosomal RNA): Single stranded, metabolically stable, makeup the two subunits of ribosomes. It constitutes 80% of the total RNA. It is a polymer with varied length from 120 – 3000 nucleotides and gives ribosomes their shape. Genes for rRNA are highly conserved and employed for phylogenetic studies.
Entrance Examination Questions Solved
Choose the correct answer:
Question 1.
Who invented electron microscope? (2010 AIIMS, 2008 JIPMER)
(a) Janssen
(b) Edison
(c) Knoll and Ruska
(d) Landsteiner
Answer:
(c) Knoll and Ruska
Question 2.
Specific proteins responsible for the flow of materials and information into the cellare called …………… . (2009 AIIMS)
(a) Membrane receptors
(b) carrier proteins
(c) integral proteins
(d) none of these
Answer:
(b) carrier proteins
![]()
Question 3.
Omnis – cellula – e – cellula was given by …………… . (2007 AIIMS)
(a) Virchow
(b) Hooke
(c) Leeuwenhoek
(d) Robert Brown
Answer:
(a) Virchow
Question 4.
Which of the following is responsible for the mechanical support, protein synthesis and enzyme transport? (2007 AIIMS)
(a) cell membrane
(b) mitochondria
(c) dictyosomes
(d) endoplasmic reticulum
Answer:
(d) endoplasmic reticulum
Question 5.
Genes present in the cytoplasm of eukaryotic cells are found in …………… . (2006 AIIMS)
(a) mitochondria and inherited via egg cytoplasm
(b) lysosomes and peroxisomes
(c) Golgi bodies and smooth endoplasmic reticulum
(d) Plastids inherited via male gametes
Answer:
(a) mitochondria and inherited via egg cytoplasm
![]()
Question 6.
In which one the following would you expect to find glyoxysomes? (2005 AIIMS)
(a) Endosperm of wheat
(b) Endosperm of castor
(c) Palisade cells in leaf
(d) Root hairs
Answer:
(b) Endosperm of castor
Question 7.
A quantosome is present in …………… . (JIPMER 2012)
(a) Mitochondria
(b) Chloroplast
(c) Golgi bodies
(d) ER
Answer:
(b) Chloroplast
Question 8.
In mitochondria the enzyme cytochrome oxidase is present in …………… . (2012 JIPMER)
(a) Outer mitochondrial membrane
(b) inner mitochondrial membrane
(c) Stroma
(d) Grana
Answer:
(b) inner mitochondrial membrane
Question 9.
Which organelle is present in higher number in secretory cell? (2008 JIPMER)
(a) Mitochondria
(b) Chloroplast
(c) Nucleus
(d) Dictyosomes
Answer:
(d) Dictyosomes
Question 10.
Major site for the synthesis of lipids …………… . (2013 NEET)
(a) Rough ER
(b) smooth ER
(c) Centriole
(d) Lysosome
Answer:
(b) smooth ER
![]()
Question 11.
Golgi complex plays a major role in …………… . (2013 NEET)
(a) post translational modification of proteins and glycosidation of lipids
(b) translation of proteins
(c) Transcription of proteins
(d) Synthesis of lipid
Answer:
(a) post translational modification of proteins and glycosidation of lipids
Question 12.
Main arena of various types of activities of a cell is …………… . (2010 AIPMT)
(a) Nucleus
(b) Mitochondria
(c) Cytoplasm
(d) Chloroplast
Answer:
(c) Cytoplasm
Question 13.
The thylakoids in chloroplast are arranged in …………… . (2005 JIPMER)
(a) regular rings
(b) linear array
(c) diagonal direction
(d) stacked discs
Answer:
(d) stacked discs
Question 14.
Sequences of which of the following is used to know the phylogeny rRNA? (20022JIPMER)
(a) mRNA
(b) rRNA
(c) tRNA
(d) Hn RNA
Answer:
(b) rRNA
Question 15.
Structures between two adjacent cells which is an effective transport pathway? (2010 AIPMT)
(a) Plasmodesmata
(b) Middle lamella
(c) Secondary wall layer
(d) Primary wall layer
Answer:
(a) Plasmodesmata
Question 16.
In active transport carrier proteins are used, which use energy in the form of ATP to …………… .
(a) transport molecules against concentration gradient of cell wall
(b) transport molecules along concentration gradient of cell membrane
(c) transport molecules against concentration gradient of cell membrane
(d) transport molecules along concentration gradient of cell wall
Answer:
(c) transport molecules against concentration gradient of cell membrane
![]()
Question 17.
The main organelle involved in modification and routing of newly synthesised protein to their destinations is …………… . (AIPMT 2005)
(a) Mitochondria
(b) Glyoxysomes
(c) Spherosomes
(d) Endoplasmic reticulum
Answer:
(d) Endoplasmic reticulum
Question 18.
Algae have cell wall made up of …………… . (AIPMT 2010)
(a) Cellulose, galactans and mannans
(b) Cellulose, chitin and glucan
(c) Cellulose, Mannan and peptidoglycan
Answer:
(a) Cellulose, galactans and mannans
Samacheer Kalvi 11th Bio Botany Biomolecules Additional Questions and Answers
Question 1.
The percentage of water in the total cellular mass is …………… .
(a) 50%
(b) 60%
(c) 70%
(d) 80%
Answer:
(c) 70%
Question 2.
The metabolites which does not show any direct function in growth is called …………… metabolite.
(a) Primary
(b) Secondary
(c) Tertiary
(d) Quartemary
Answer:
(b) Secondary
Question 3.
Molecular formula for carbohydrates is …………… .
(a) (CH2O)2
(b) (CH6O)
(C) (C2H2O)n
(d) (CH6O)n
Answer:
(a) (CH2O)2
Question 4.
Number of carbon molecule in glucose is …………… .
(a) 4
(b) 6
(c) 8
(d) 12
Answer:
(b) 6
![]()
Question 5.
Number of sugar units in oligo saccharides are …………… .
(a) 6 to 10
(b) 1 to 10
(c) 2 to 8
(d) 2 to 10
Answer:
(d) 2 to 10
Question 6.
Which of the following is a trisaccharide?
(a) Maltose
(b) Stachyose
(c) Ramnose
(d) Aldose
Answer:
(c) Ramnose
Question 7.
…………… are also called as Glycan.
(a) Monosaccharides
(b) Disaccharides
(c) Polysaccharides
(d) Multisaccharides
Answer:
(c) Polysaccharides
Question 8.
Sucrose is a combination of …………… and fructose.
(a) α – glucose
(b) β – glucose
(c) Ketoses
(d) Maltose
Answer:
(a) α – glucose
![]()
Question 9.
…………… is also called as animal starch.
(a) Amylose
(b) Glycogen
(c) Glucose
(d) Glycerol
Answer:
(b) Glycogen
Question 10.
…………… reagent is used in starch test.
(a) Potassium permanganate
(b) Potassium iodide
(c) Calcium chloride
(d) Calcium iodide
Answer:
(b) Potassium iodide
Question 11.
Glycogen is not seen in …………… cells.
(a) liver
(b) skeletal
(c) muscle
(d) brain
Answer:
(d) Brain
Question 12.
Benedicts solution is nothing but …………… .
(a) Copper II sulphate
(b) Cuprous sulphate
(c) Cupric sulphate
(d) Copper I sulphate
Answer:
(a) Copper II sulphate
Question 13.
…………… is not a reducing sugar.
(a) Glucose
(b) Fructose
(c) Sucrose
(d) Ketose
Answer:
(c) Sucrose
![]()
Question 14.
…………… form the exoskeleton of insects & arthropods.
(a) N – acetyl glucosamine
(b) N – butyl glucosamine
(c) N – phenyl glucosamine
(d) N – methyl glucosamine
Answer:
(a) N – acetyl glucosamine
Question 15.
Number of fatty acids in triglyceride is …………… .
(a) 1
(b) 2
(c) 3
(d) 4
Answer:
(c) 3
Question 16.
The major structural component of cell membrane is …………… .
(a) glucolipids
(b) phospholipids
(c) proteolipids
(d) triglycerides
Answer:
(b) phospholipids
Question 17.
There are …………… different amino acids existing naturally.
(a) about 20
(b) about 10
(c) about 25
(d) about 22
Answer:
(a) about 20
Question 18.
A zwitterion also called as …………… ion.
(a) dipolar
(b) monopolar
(c) tripolar
(d) nonpolar
Answer:
(a) dipolar
Question 19.
…………… test is used as an indicator of the presence of protein.
(a) Biuret test
(b) Iodine test
(c) Benedict’s test
(d) Starch test
Answer:
(a) Biuret test
![]()
Question 20.
The competitive inhibitor is …………… for succinic dehydrogenase.
(a) malonate
(b) succinate
(c) oxalate
(d) citrate
Answer:
(a) malonate
Question 21.
…………… is the abundant protein in whole biosphere.
(a) RUBP
(b) NAD+
(c) NADPH
(d) RUBISCO
Answer:
(d) RUBISCO
Question 22.
…………… is an active enzyme with its non – protein component.
(a) Apoenzyme
(b) Holoenzyme
(c) Coenzymes
(d) Enzymes
Answer:
(b) Holoenzyme
Question 23.
Flavin adenine dinucleotide contains …………… which helps to accept hydrogen.
(a) ascolac acid
(b) cyanocobalamin
(c) riboflavin
(d) keratinine
Answer:
(c) riboflavin
Question 24.
…………… is a catalytic RNA.
(a) mRNA
(b) Ribozyme
(c) Ribonuclease
(d) rRNA
Answer:
(b) Ribozyme
![]()
Question 25.
…………… protects the end of the chromosomes from damage.
(a) Satellite
(b) Kinetochore
(c) Primary constriction
(d) Telomere
Answer:
(d) Telomere
Question 26.
Which is not a pyrimidine base?
(a) Cytosine
(b) Uracil
(c) Guanine
(d) Thymine
Answer:
(c) Guanine
Question 27.
Which type of DNA was described by Watson & Crick?
(a) Z – DNA
(b) α – DNA
(c) B – DNA
(d) A – DNA
Answer:
(c) B – DNA
Question 28.
According to Chargaff’s rule, the hydrogen bonding between Adenine and Thymine is …………… .
(a) 2
(b) 3
(c) 4
(d) Nil
Answer:
(a) 2
Question 29.
The first clear crystallographic evidence for helical structure of DNA was produced by …………… .
(a) Maurice Wilkins
(b) Rosalind Franklin
(c) Francis Crick
(d) Chargaff
Answer:
(b) Rosalind Franklin
![]()
Question 30.
According to Cargaff’s rule, A : T = G : C = …………… .
(a) 0
(b) 1
(c) >1
(d) <1
Answer:
(b) 1
Question 31.
A complete turn of the helix comprises …………… .
(a) 34 nm
(b) 3.4 nm
(c) 20 nm
(d) 2nm
Answer:
(b) 3.4 nm
Question 32.
Diameter of DNA helix is …………… .
(a) 34 Å
(b) 20 nm
(c) 34 nm
(d) 20 Å
Answer:
(d) 20 Å
Question 33.
RNA is …………… .
(a) Single stranded and stable
(b) Single stranded and unstable
(c) Double stranded and stable
(d) Double stranded and unstable
Answer:
(b) Single stranded and unstable
Question 34.
rRNA constitutes …………… of total RNA.
(a) 20%
(b) 70%
(c) 80%
(d) 15%
Answer:
(c) 80%
Question 35.
Shape to the ribosomes is provided by …………… .
(a) rRNA
(b) tRNA
(c) mRNA
(d) DNA
Answer:
(a) rRNA
Question 36.
Which RNA is also called as soluble RNA?
(a) rRNA
(b) tRNA
(c) mRNA
(d) ssRNA
Answer:
(b) tRNA
Question 37.
Which is the left – handed DNA?
(a) B – DNA
(b) A – DNA
(c) Z – DNA
(d) dsDNA
Answer:
(c) Z – DNA
![]()
Question 38.
Which of the following does not contain cell wall?
(a) Fungi
(b) Bacteria
(c) Mycoplasma
(d) Algae
Answer:
(c) Mycoplasma
Question 39.
The amino acid which is both an acid and a base is called …………… .
(a) Amphibolic
(b) Amphoteric
(c) Amphipathetic
(d) Anabolic
Answer:
(b) Amphoteric
Question 40.
…………… leads to the loss of 3D structure of protein.
(a) Annealing
(b) Extension
(c) Denaturation
(d) Polymerisation
Answer:
(c) Denaturation
Question 41.
Which of the following polysaccharides is used as solidifying agent in culture medium?
(a) Inulin
(b) Heparin
(c) Agar
(d) Keratan sulphate
Answer:
(c) Agar
Question 42.
Which is an anticoagulant?
(a) Inulin
(b) Heparin
(c) Agar
(d) Keratan sulphate
Answer:
(b) Heparin
Question 43.
Insulin is a polymer of …………… .
(a) sucrose
(b) fructose
(c) glucose
(d) maltose
Answer:
(b) fructose
II. Very Short Answer Type Questions (2 Marks)
Question 1.
Define cell pool and mention its constituents.
Answer:
The cell components are made of collection of molecules called as cellular pool, which consists of both inorganic and organic compounds.
Question 2.
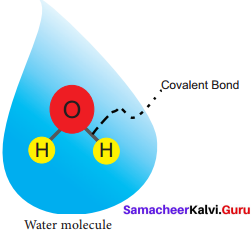
Draw the molecular structure of water.
Answer:
the molecular structure of water:
Question 3.
Point out the percentage of water in human cell & a plant cell.
Answer:
Water makes upto 70% of human cell and upto 95% of mass of a plant cell.
Question 4.
What are metabolites?
Answer:
Metabolites are the organic compounds synthesized by plants, fungi and various microbes. They are the intermediates & products of metabolism.
![]()
Question 5.
Write the molecular formula for carbohydrates?
Answer:
(CH2O)n
Question 6.
Give an example for simple sugar with its formula.
Answer:
Glucose – C6H12O6
Question 7.
Which type of sugar does sucrose belongs to? Write its monomer units.
Answer:
Sucrose is a disaccharides composed of α – glucose & fructose.
Question 8.
Classify polysaccharides based on function.
Answer:
Depending on the function, polysaccharides are of two types:
- storage polysaccharide and
- structural polysaccharide.
Question 9.
What are Glycans?
Answer:
Polysaccharides are also called as Glycans. They are made of hundreds of monosaccharide units.
Question 10.
Which is a common storage polysaccharide? Mention its monomer units.
Answer:
Starch is a storage polysaccharide made up of repeated units of amylose and amylopectin.
![]()
Question 11.
Which is an animal starch? Where can we see it in our body?
Answer:
Glycogen. It is found in liver cells & skeletal muscles.
Question 12.
Why oil does not get mixed if added with water?
Answer:
Oil is a lipid. Lipids are long hydrocarbon chains that are non-polar & thus hydrophobic, which avoids the oil to dissolve in water.
Question 13.
How saturated fatty acids differ from unsaturated fatty acids?
Answer:
Saturated factty acids have the hydrocarbon chain with single bond, whereas in unsaturated fatty acids the hydrocarbon chain will have double bonds.
![]()
Question 14.
How waxes are formed?
Answer:
Waxes are esters formed between a long chain alcohol and saturated fatty acids.
Question 15.
Why amino acids are amphoteric?
Answer:
The amino acid is both an acid and a base and is called amphoteric.
Question 16.
Name the various groups attached to the 4 valencies of carbon in an amino acid.
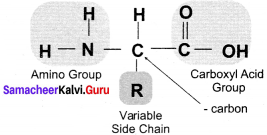
Answer:
The 4 valencies of carbon in an amino acid:
- (NH2)
- an acidic carboxylic group (COOH) and
- a hydrogen atom (H)
- and side chain or variable R group.
Question 17.
Where the peptide bond is formed?
Answer:
A peptide bond is formed when the amino group of one amino acid reacts with carbonyl group of another amino acid.
![]()
Question 18.
Which was the first sequenced protein? Who had done it?
Answer:
First protein is insulin and it was sequenced by Fred Sanger.
Question 19.
Why proteins undergo conformational changes after its synthesis?
Answer:
After synthesis, the protein attains conformational change into a specific 3D form for proper functioning.
Question 20.
Mention the levels of protein organisation based on folding.
Answer:
According to the mode of folding, four levels of protein organisation have been recognised namely primary, secondary, tertiary and quaternary.
Question 21.
Define enzymes.
Answer:
Enzymes are globular proteins that catalyse the thousands of metabolic reactions taking place within cells and organism.
![]()
Question 22.
Name any four factors that affect enzyme reactions.
Answer:
Four factors that affect enzyme reactions:
- pH
- temperature
- enzyme concentration and
- substrate concentration.
Question 23.
What are inhibitors? Mention its types.
Answer:
Certain substances present in the cells may react with the enzyme and lower the rate of reaction. These substances are called inhibitors. It is of two types:
- Competitive and
- Non – competitive.
Question 24.
Differentiate Apoenzyme from Holoenzyme.
Answer:
Differ Apoenzyme from Holoenzyme:
| Apoenzyme |
Holoenzyme |
| 1. Active enzyme with its non – protein component | 1. Inactive enzyme without its non – protein component |
Question 25.
What are Prosthetic groups? Give example.
Answer:
Prosthetic groups are organic molecules that assist in catalytic function of an enzyme. Example: Flavin adenine dinucleotide (FAD).
Question 26.
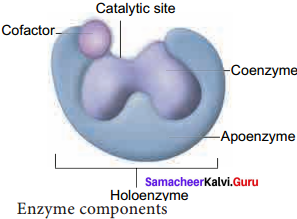
Draw a diagram showing the various components of enzymes.
Answer:
Catalytic site, Cofactor and Holoenzyme:
Question 27.
Write a note on Ribozyme.
Answer:
Ribozyme – Non – Protein Enzyme. A Ribozyme, also called as catalytic RNA; is a ribonucleic acid that acts as enzyme. It is found in ribosomes.
![]()
Question 28.
Give an example for following enzyme groups.
Answer:
An example for following enzyme groups:
- Transferase – Ex: Transaminase
- Isomerase – Ex: Isomerase
- Oxidoreductase – Ex: Dehydrogenase
- Lyase – Ex: Decarboxylase
Question 29.
Write the composition of DNA & RNA.
Answer:
Nitrogen base, pentose sugar and phosphate.
Question 30.
What is a nucleoside?
Answer:
A purine or a pyrimidine and a ribose or deoxyribose sugar is called nucleoside. A nitrogenous base is linked to pentose sugar through n-glycosidic linkage and forms a nucleoside.
Question 31.
What is a nucleotide?
Answer:
When a phosphate group is attached to a nucleoside it is called a nucleotide.
Question 32.
Name the two types of Purines and Pyrimidines.
Answer:
The two types of Purines and Pyrimidines:
- Purines: Adenine and guanine
- Pyrimidines: Cytosine and thymine (Uracil)
Question 33.
How DNA differs from RNA?
Answer:
DNA has thymine base, whereas RNA has uracil base. DNA has deoxyribose sugar, whereas RNA has ribose sugar.
Question 34.
Draw a simple diagram showing basic components of DNA.
Answer:
Deoxyribose sugar:
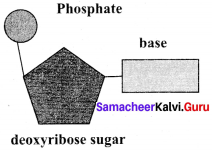
Question 35.
Which is the secondary structure of DNA? Who discovered it?
Answer:
B – DNA is the secondary structure of DNA. Watson & Crick discovered B – DNA.
Question 36.
State Chargaff’s rule.
Answer:
Chargaff’s Rule:
A = T; G = C
A + G = T + C,
A : T = G : C = 1.
Question 37.
Name the three forms of DNA.
Answer:
The three forms of DNA:
- A – DNA
- B – DNA and
- Z – DNA.
Question 38.
Which is the soluble forms of RNA. Write its percentage composition of total RNA.
Answer:
tRNA is the soluble RNA which is about 15% of total RNA.
![]()
Question 39.
Name the types of RNA?
Answer:
The types of RNA:
- mRNA
- tRNA and
- rRNA.
Question 40.
Draw the structure of transfer RNA.
Answer:
Transfer RNA (tRNA):
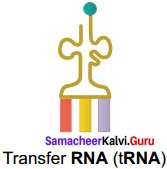
III. Short Answer Type Questions (3 Marks)
Question 1.
Distinguish between Macronutrients & Micronutrients.
Answer:
Macronutrients:
- Nutrients required in larger quantities for plant growth are called Macronutrients.
- e.g. Potassium and Calcium
Micronutrients:
- Nutrients required in trace amount for plant growth are called Micronutrients
- e.g. Zinc and Bora
Question 2.
Tabulate the various cellular components with their percentage.
Answer:
The various cellular components with their percentage:
| Component |
% of the total cellular mass |
| 1. Water | 1. 70 |
| 2. Proteins | 2. 15 |
| 3. Carbohydrates | 3. 3 |
| 4. Lipids | 4. 2 |
| 5. Nucleic acids | 5. 6 |
| 6. Ions | 6. 4 |
Question 3.
List out the properties of Water.
Answer:
Properties of Water:
- Adhesion and cohesion property
- High latent heat of vaporisation
- High melting and boiling point
- Universal solvent
- Specific heat capacity
Question 4.
How lattice formation occurs in water molecule?
Answer:
Two electro negative atoms of oxygen share a hydrogen bonds of two water molecule. Thus, they can stick together by cohesion and results in lattice formation.
![]()
Question 5.
Distinguish between Primary metabolite & Secondary metabolite.
Answer:
Between Primary metabolite & Secondary metabolite:
- Primary metabolites are those that are required for the basic metabolic processes like photosynthesis, respiration, etc Example: Lipase, a protein.
- Secondary metabolites does not show any direct function in growth and development of organisms. Example: Ricin, gums.
Question 6.
Define Polymerization.
Answer:
Polymerization, is a process in which repeating subunits termed monomers are bound into chains of different lengths. These chains of monomers are called polymers.
Question 7.
Explain the bond formation in sucrose molecule.
Answer:
Sucrose is formed from a molecule of α – glucose and a molecule of fructose. This is a condensation reaction releasing water. The bond formed between the glucose and fructose molecule by removal of water is called glycosidic bond. This is another example of strong, covalent bond.
![]()
Question 8.
How will you identify the presence of starch in a food sample.
Answer:
The presence of starch is identified by adding a solution of iodine in potassium iodide. Iodine molecules fit nearly into the starch helix, creating a blue – black colour.
Question 9.
Write a note on steroids.
Answer:
Steroids are complex compounds commonly found in cell membrane and animal hormones. e.g. Cholesterol which reinforces the structure of the cell membrane in animal cells and in an unusual group of cell wall deficient bacteria – Mycoplasma.
Question 10.
Draw the structure of basic amino acid.
Answer:
The structure of basic amino acid:
Question 11.
What is a Zwitterion? or What is an isoelectric point?
Answer:
A zwitterion also called as dipolar ion, is a molecule with two or more functional groups, of which at least one has a positive and other has a negative electrical charge and the net charge of the entire molecule is zero. The pH at which this happens is known as the isoelectric point.
Question 12.
Write briefly about protein denaturation.
Answer:
Denaturation is the loss of 3D structure of protein. Exposure to heat causes atoms to vibrate violently, and this disrupts the hydrogen and ionic bonds. Under these conditions, protein molecules become elongated, disorganised strands. Agents such as soap, detergents, acid, alcohol and some disinfectants disrupt the interchain bond and cause the molecule to be non – functional.
Question 13.
Why do some people have curly hair?
Answer:
Human hair is made of protein. The more the distance between the sulphur atoms, the more the proteins bend; the more the hair curls.
![]()
Question 14.
Write a note on RUBISCO.
Answer:
Ribulose biphosphate carboxylase oxygenase (RUBISCO) is an enzyme that catalyses the reaction between CO2 and the CO2 acceptor molecule in photosynthesis. It is the most abundant protein in the whole biosphere.
Question 15.
Differentiate Anabolic reaction and Catabolic reaction.
Answer:
Anabolic reaction:
- Anabolic reaction involves the building up of organic molecules.
- Ex: Synthesis of protein from amino acids.
Catabolic reaction:
- Catabolic reaction involves the breaking down of larger molecules.
- Ex: Breaking down of sugar in respiration.
Question 16.
What are Allosteric inhibitors?
Answer:
Compounds which modify enzyme activity by causing a reversible change in the structure of the enzyme active site. This in turn affects the ability of the substrate to bind to the enzyme. Such compounds are called allosteric inhibitors, e.g. The enzyme hexokinase which catalysis glucose to glucose – 6 phosphate in glycolysis is inhibited by glucose – 6 phosphate. This is an example for feedback allosteric inhibitor.
Question 17.
Explain in brief about End – product inhibitor. (Negative Feedback Inhibition)
Answer:
When the end product of a metabolic pathway begins to accumulate, it may act as an allosteric inhibitor of the enzyme controlling the first step of the pathway. Thus the product starts to switch off its own production as it builds up. The process is self – regulatory. As the product is used up, its production is switched on once again. This is called end – product inhibition.
![]()
Question 18.
Draw the structure of Purine & Pyrimidine.
Answer:
The structure of Pyrimidine & Purine:
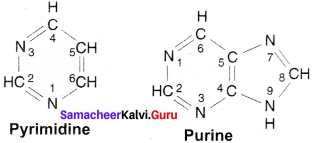
Question 19.
Why the sugar in DNA is a deoxyribose?
Answer:
The sugar in DNA molecule is called 2’ – deoxyribose because there is no hydroxyl group at 2’ position.
Question 20.
How dinucleotide & polynucleotides are formed?
Answer:
Two nucleotides join to form dinucleotide that are linked through 3′ – 5′ phosphodiester linkage by condensation between phosphate groups of one with sugar of other. This is repeated many times to make polynucleotide.
Question 21.
Compare Plectonemic & Paranemic Coiling.
Answer:
Plectonemic coiling – the two strands of the DNA are wrapped around each other in a helix, making it impossible to simply move them apart without breaking the entire structure. Whereas in Paranemic coiling the two strands simply lie alongside one another, making them easier to pull apart.
![]()
Question 22.
Differentiate between Polycistronic & Monocistronic mRNA.
Answer:
Between Polycistronic & Monocistronic mRNA:
|
Polycistronic mRNA |
Monocistronic mRNA |
| 1. Polycistronic mRNA carry coding sequences for many Polypeptides | 1. Monocistronic mRNA carry coding sequences for only one Polypeptide |
| 2. Prokaryotic mRNA are polycistronic | 2. Eukaryotic mRNA are monocistronic |
Question 23.
What are Proteins?
Answer:
Proteins are polymers of 20 different amino acids, each of which has a distinct side chain with specific chemical properties. Each protein has a unique amino acid sequence which determines its 3D structure.
Question 24.
Herbivores can digest cellulose rich food, Why can’t human beings?
Answer:
Human cannot digest cellulose but herbivores can digest them with the help of bacteria present in the gut which produces enzymes cellulase. This is an example of mutualism.
![]()
Question 25.
How will you identify the presence of protein in food samples?
Answer:
The biuret test is used as an indicator of the presence of protein because it gives a purple colour in the presence of peptide bonds (-C- N-). To a protein solution an equal quantity of sodium hydroxide solution is added and mixed. Then a few drops of 0.5% copper (II) sulphate is added with gentle mixing. A distinct purple colour develops without heating.
Question 26.
Write a note on peptide bonds between amino acids.
Answer:
The amino group of one amino acid reacts with carboxyl group of other amino acid, forming a peptide bond. Two amino acids can react together with the loss of water to form a dipeptide. Long strings of amino acids linked by peptide bonds are called polypeptides. In 1953, Fred Sanger first sequenced the Insulin protein.
Question 27.
Which was the first alkaloid discovered? Mentions its uses.
Answer:
Morphine is the first alkaloid to be found. It comes from the plant Opium poppy (Papaver somniferum). It is used as a pain reliever in patients with severe pain levels and cough suppressant.
IV. Long Answer Type Questions (5 Marks)
Question 1.
How will you identify the presence of glucose in a given food sample?
Answer:
Aldoses and ketoses are reducing sugars. This means that, when heated with an alkaline solution of copper (II) sulphate (a blue solution called Benedict’s solution), the aldehyde or ketone group reduces Cu2+ ions to Cu+ ions forming brick red precipitate of copper (I) oxide. In the process, the aldehyde or ketone group is oxidised to a carboxyl group (-COOH).
This reaction is used as test for reducing sugar and is known as Benedict’s test. The results of Benedict’s test depends on concentration of the sugar. If there is no reducing sugar it remains blue. Sucrose is not a reducing sugar The greater the concentration of reducing sugar, the more is the precipitate formed and greater is the colour change.
![]()
Question 2.
Write a note on various levels of protein organisation.
Answer:
The primary structure is linear arrangement of amino acids in a polypeptide chain. Secondary structure arises when various functional groups are exposed on outer surface of the molecular interaction by forming hydrogen bonds. This causes the amino acid chain to twist into coiled configuration called α – helix or to fold into a flat β – pleated sheets.
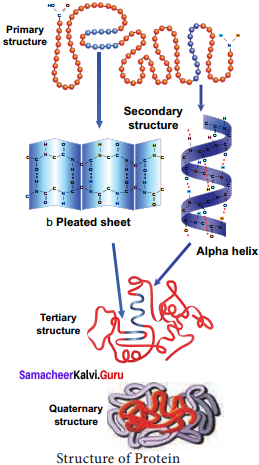
Tertiary protein structure arises when the secondary level proteins fold into globular structure called domains. Quaternary protein structure may be assumed by some complex proteins in which more than one polypeptide forms a large multiunit protein. The individual polypeptide chains of the protein are called subunits and the active protein itself is called a multimer.
Question 3.
Enumerate the properties of Enzyme.
Answer:
The properties of Enzyme:
- Enzymes are globular proteins.
- They act as catalysts and effective even in small quantity.
- They remain unchanged at the end of the reaction.
- They are highly specific.
- They have an active site where the reaction takes place.
- Enzymes lower activation energy of the reaction they catalyse.
Question 4.
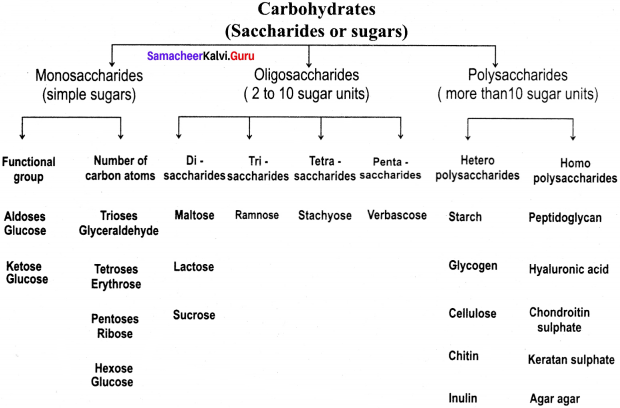
Draw a Flow Chart depicting the Carbohydrate Classification
Answer:
Flow Chart depicting the Carbohydrate Classification:
Question 5.
Explain the various types of chemical bonding in proteins.
Answer:
1. Hydrogen Bond: It is formed between some hydrogen atoms of oxygen and nitrogen in polypeptide chain. The hydrogen atoms have a small positive charge and oxygen and nitrogen have small negative charge. Opposite charges attract to form hydrogen bonds. Though these bonds are weak, large number of them maintains the molecule in 3D shape.
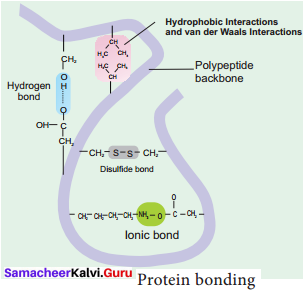
2. Ionic Bond: It is formed between any charged groups that are not joined together by peptide bond. It is stronger than hydrogen bond and can be broken by changes in pH and temperature.
3. Disulfide Bond: Some amino acids like cysteine and methionine have sulphur. These form disulphide bridge between sulphur atoms and amino acids.
4. Hydrophobic Bond: This bond helps some protein to maintain structure. When globular proteins are in solution, their hydrophobic groups point inwards away from water.
Question 6.
Explain Lock & Key Mechanism of Enzymatic reaction.
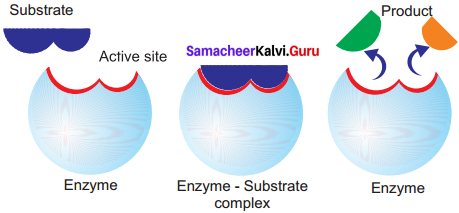
Answer:
Lock and Key Mechanism of Enzyme: In a enzyme catalysed reaction, the starting substance is the substrate. It is converted to the product. The substrate binds to the specially formed pocket in the enzyme – the active site, this is called lock and key mechanism of enzyme action. As the enzyme and substrate form a ES complex, the substrate is raised in energy to a transition state and then breaks down into products plus unchanged enzyme.
Question 7.
Describe the Competitive & Non – Competitive Inhibitors of enzyme.
Answer:
1. Competitive Inhibitor: Molecules that resemble the shape of the substrate and may compete to occupy the active site of enzyme are known as competitive inhibitors. For Example: the enzyme that catalyses the reaction between carbon dioxide and the CO2 acceptor molecule in photosynthesis, known as ribulose biphosphate carboxylase oxygenase (RUBISCO) is competitively inhibited by oxygen / carbon – di – oxide in the chloroplast. The competitive inhibitor is malonate for succinic dehydrogenase.
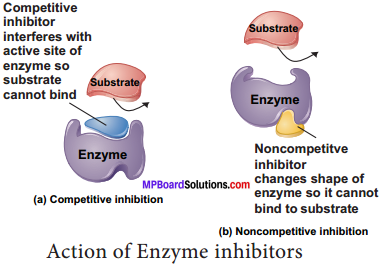
2. Non – competitive Inhibitors: There are certain inhibitors which may be unlike the substrate molecule but still combines with the enzyme. This either blocks the attachment of the substrate to active site or change the shape so that it is unable to accept the substrate. For example the effect of the amino acids alanine on the enzyme pyruvate kinase in the final step of glycolysis.
Certain non – reversible / irreversible inhibitors bind tightly and permanently to an enzyme and destroy its catalytic properties entirely. These could also be termed as poisons. Example – cyanide ions which blocks cytochrome oxidase in terminal oxidation in cell aerobic respiration, the nerve gas sarin blocks a neurotransmitter in synapse transmission.
Question 8.
Give a detailed account on Enzyme Co – factors.
Answer:
Many enzymes require non – protein components called co – factors for their efficient activity. Co – factors may vary from simple inorganic ions to complex organic molecules.
They are of three types:
- Inorganic ions, prosthetic groups and coenzymes.
- Holoenzyme – active enzyme with its non – protein component.
- Apoenzyme – the inactive enzyme without its non – protein component.
Inorganic ions help to increase the rate of reaction catalysed by enzymes. Example: Salivary amylase activity is increased in the presence of chloride ions. Prosthetic groups are organic molecules that assist in catalytic function of an enzyme. Flavin adenine dinucleotide (FAD) contains riboflavin(vit B2), the function of which is to accept hydrogen. ‘Haem’ is an iron – containing prosthetic group with an iron atom at its centre. Coenzymes are organic compounds which act as cofactors but do not remain attached to the enzyme. The essential chemical components of many coenzymes are vitamins. Eg. NAD, NADP, Coenzyme A, ATP.
Question 9.
Tabulate the various features of different forms of DNA.
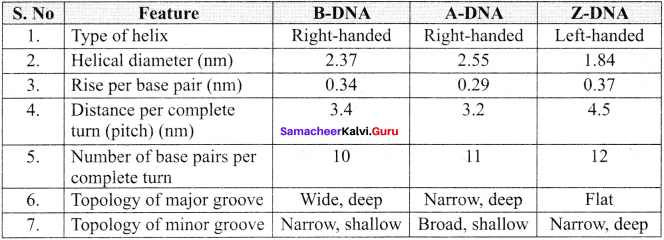
Answer:
The various features of different forms of DNA:
Question 10.
Compare DNA with RNA?
Answer:
Compare DNA with RNA:
| DNA |
RNA |
| 1. Deoxyribose sugar is present | 1. Ribose sugar is present |
| 2. Thymine is present | 2. Uracil is present |
| 3. More stable | 3. Less stable |
| 4. Double stranded | 4. Single stranded |
| 5. Types: A – DNA, B – DNA, Z – DNA | 5. Type: mRNA, tRNA, rRNA |
| 6. Genetic material for most of living organism except few viruses | 6. Genetic material for few viruses only |
V. Higher Order Thinking Skills (HOTs)
Question 1.
In which form does the glucose is stored in animal cells? Specify the cells?
Answer:
Glucose is stored in the form of glycogen. Glycogen is stored in liver cells and skeletal muscles, etc.
Question 2.
State the key differences between DNA & RNA.
Answer:
The key differences between DNA & RNA:
| DNA |
RNA |
| 1. Double stranded | 1. Single stranded |
| 2. Thymine is the pyrimidine base | 2. Uracil is the pyrimidine base |
![]()
Question 3.
Aminoacids are the monomers of proteins. Similarly mention the monomers of nucleic acids along with its composition.
Answer:
The monomer unit of nucleic acids are nucleotides, which are composed of nitrogen base, pentose sugar and phosphoric acid.
Question 4.
Complete the equations.
(a) Nitrogen base + …………… . = Nucleoside.
(b) …………… + nucleoside = Nucleotide.
(c) Glucose + fructose = …………… .
Answer:
(a) sugar
(b) phosphoric acid and
(c) sucrose.
Question 5.
What happens if the sucrose is hydrolysed?
Answer:
On hydrolysis, the glycosidic bonds in sucrose gets splitted yielding glucose and fructose.
![]()
Question 6.
Name the types of bonds.
(a) Between amino acids of protein
(b) Between carboxyl group and glycerol of fatty acids and
(c) Between glucose units of cellulose.
Answer:
(a) Peptide bond
(b) Ester bond
(c) Glycosidic bond
Question 7.
Study the following equation and name the reaction A and B.
![]()
Answer:
Reaction A is glycogenolysis. Reaction B is glycogenesis.
Question 8.
Whether waxes are found in living organisms?
Answer:
Yes. Fur, feathers, fruits, leaves, skin, and exoskeleton of insects are naturally water – proofed with a coating of wax.
![]()
Question 9.
If dsDNA has 40% Guanine. Calculate the percentage of Adenine.
Answer:
According to Chargaff’s rule:
Guanine pairs with cytosine. It Guanine is 40%, then cytosine will also be 40%. Similarly, Adenine pairs with thymine, if guanine is 40%, the remaining 60% will be Adenine. So Thymine will also be 60%.
Thus, A : T = G : C = 1
and 60 : 60 = 40 : 40 = 1.
Question 10.
In an Eukaryotic cell, totally there are 10000 RNA molecules. Calculate the number of mRNA’s and tRNA’s if the count of rRNA is 8000.
Answer:
In a cell, rRNA contributes 80%, tRNA constitutes 15% and mRNA constitutes 5%. If rRNA is 8000 (80%), then tRNA count is 1500 (15%) and mRNA is 500 (5%).
Question 11.
Despite made of two different monomers amylose and amylopectin, starch is a homopolysaccharide – Comment.
Answer:
Starch is made up of amylose and amylopectin. Both are glucose polymers, hence starch is considered as homopolysaccharides.
![]()
Question 12.
How do you call a fatty acid as saturated or unsaturated?
Answer:
If the hydrocarbon chain is single bonded, then the fatty acid is said to be saturated. In unsaturated fatty acids, the hydrocarbon chain is double bonded.
Question 13.
Enzymes are biocatalysts – Justify.
Answer:
Enzymes are globular proteins that catalyze thousands of metabolic reactions taking place within cells and organisms. Hence enzymes are called as biological catalysts.
Question 14.
Starch, cellulose, glycogen and chitin are polysaccharides found among the following. Choose the one appropriate and write against each.
(a) Cotton fibre – …………… .
(b) Exoskeleton of ant – …………… .
(c) Liver – …………… .
(d) Peeled potato – …………… .
Answer:
(a) Cellulose
(b) Chitin
(c) Glycogen and
(d) Starch.
![]()
Question 15.
Sucrose is not a reducing sugar. Why?
Answer:
Sucrose is a non – reducing sugar since it does not possess aldehyde or ketone group, which is responsible for reducing the alkaline solutions like copper (II) sulphate.
Question 16.
A DNA segment has a total of 1000 nucleotides, out of which 240 are adenine containing nucleotides. How many pyrimidine bases this DNA segment possess?
Answer:
Pyrimidine = 500.
According to Chargaff’s rule,
A = T,
A = 240, hence T = 240.
A + T = 240 + 240 = 480.
So, G + C = 1000 – 480 = 520.
G = C, Therefore, C = \(\frac {520}{2}\) = 260.
Thus, pyrimidine = C + T = 260 + 240 = 500.