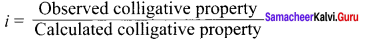Students can Download Chemistry Chapter 9 Solutions Questions and Answers, Notes Pdf, Samacheer Kalvi 11th Chemistry Solutions Guide Pdf helps you to revise the complete Tamilnadu State Board New Syllabus and score more marks in your examinations.
Tamilnadu Samacheer Kalvi 11th Chemistry Solutions Chapter 9 Solutions
Samacheer Kalvi 11th Chemistry Chapter 9 Solutions Textual Evaluation Solved
Samacheer Kalvi 11th Chemistry Solutions Multiple Choice Questions
Question 1.
The molality of a solution containing 1 .8g of glucose dissolved in 250g of water is …………
(a) 0.2 M
(b) 0.01 M
(c) 0.02 M
(d) 0.04 M
Answer:
(d) 0.04 M
Solution:
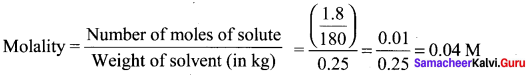
Question 2.
Which of the following concentration terms is/are independent of temperature?
(a) molality
(b) molarity
(c) mole fraction
(d) (a) and (c)
Answer:
(d) (a) and (c)
Solution:
Molality and mole fraction are independent of temperature.
![]()
Question 3.
Stomach acid, a dilute solution of HCI can be neutralised by reaction with Aluminium hydroxide
Al(OH)3 + 3HCl(aq) → AlCl3 + 3H2O
How many millilitres of 0.1 M Al(OH)3 solution are needed to neutralise 21 mL of 0.1 M HCl
(a) 14 mL
(b) 7 mL
(c) 21 mL
(d) none of these
Answer:
(b) 7 mL
Solution:
M1 x V1 = M2 x V2
∵ 0.1 M Al(OH)3 gives 3 x 0.1 = 0.3 M OH– ions .
0.3 x V1 = 0.1 x 21
V1= \(\frac { 0.1 x 21 }{ 0.3 }\) = 7ml
Question 4.
The partial pressure of nitrogen in air is 0.76 atm and its Henry’s law constant is 7.6 x 104 atm at 300K. What is the mole fraction of nitrogen gas in the solution obtained when air is bubbled through water at 300K?
(a) 1 x 10-4
(b) 1 x 10-6
(c) 2 x 10-5
(d) 1 x 10-5
Answer:
(d) 1 x 10-5
Solution:
PN2 = 0.76atm
KH = 7.6 x 104
x = ?
PN2 = KH . x
0.76 = 7.6 x 104x x
x = \(\frac { 0.76 }{ 7.6\times { 10 }^{ 4 } }\) = 1 x 10-5
![]()
Question 5.
The Henry’s law constant for the solubility of Nitrogen gas in water at 350K is 8 x 104 atm. The mole fraction of nitrogen in air is 0.5. The number of moles of Nitrogen from air dissolved in 10 moles of water at 350K and 4 atm pressure is ………….
(a) 4 x 10-4
(b) 4 x 104
(c) 2 x 10-2
(d) 2.5 x 10-4
Answer:
(d) 2.5 x 10-4
Solution:
KH = 8 x 104
(xN2 )in air = 0.5
Total pressure = 4 atm
Partial pressure of nitrogen = Mole fraction Total pressure
= O.5 x 4 = 2
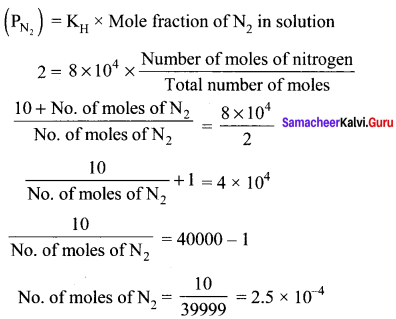
Question 6.
Which one of the following is incorrect for ideal solution?
(a) ∆Hmix = 0
(b) ∆Umix = o
(c) ∆P = PObserved – PCalculated by raoults law = 0
(d) ∆Gmix = 0
Answer:
(d) ∆Gmix = 0
Solution:
For an ideal solution, ∆Smix \(\neq\) 0; Hence ∆Gmix \(\neq\) 0
∴ Incorrect is ∆Gmix = 0
Question 7.
Which one of the following gases has the lowest value of Henry’s law constant?
(a) N2
(b) He
(c) CO2
(d) H2
Answer:
(c) CO2
Solution:
Carbon dioxide; most stable gas and has lowest value of Henry’s Law constant.
Question 8.
P1 and P2 are the vapour pressures of pure liquid components, 1 and 2 respectively of an ideal binary solution if x1 represents the mole fraction of component 1, the total pressure of the solution formed by 1 and 2 will be ………
(a) P1 + x1(P2 – P1)
(b) P2 – x1(P2 + P1)
(c) P1 – x2(P1 – P2)
(d) P1 + x2(P1 – P2)
Answer:
(c) P1 – x2(P1 – P2)
Solution:
Ptotal = P1 + P2
= P1 x1 + P2x2
= P1(1 – x2) + P2x2
= P1 – P1x2 + P2x2 = P1 – x2(P1 – P2)
[∵x1 + x2 = 1
x1 = 1 – x2]
![]()
Question 9.
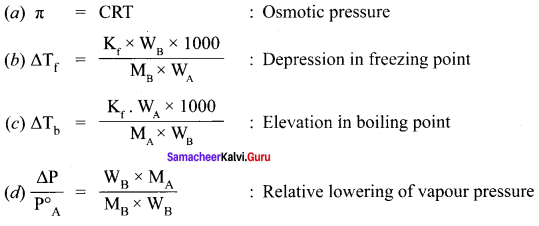
Osomotic pressure (π) of a solution is given by the relation ……………
(a) π = nRT
(b) πV = nRT
(c) πRT = n
(d) none of these
Answer:
(b) πV = nRT
Solution:
n = CRT
n = \(\frac { n }{ V }\)
π V = nRT
Question 10.
Which one of the following binary liquid mixtures exhibits positive deviation from Raoults law?
(a) Acetone + chloroform
(b) Water + nitric acid
(c) HCI + water
(d) ethanol + water
Answer:
(d) ethanol + water
Question 11.
The Henry’s law constants for two gases A and B are x and y respectively. The ratio of mole fractions of A to B is 0.2. The ratio of mole fraction of B and A dissolved in water will be …………
(a) \(\frac { 2x }{ y }\)
(b) \(\frac { y }{ 0.2x }\)
(c) \(\frac { 0.2x }{ y }\)
(d) \(\frac { 5x }{ y }\)
Answer:
(d) \(\frac { 5x }{ y }\)
Solution:
Given,
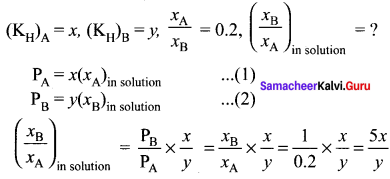
Question 12.
At 100°C the vapour pressure of a solution containing 6.5g a solute in 100g water is 732mm. If Kb = 0.52, the boiling point of this solution will be …………..
(a) 102°C
(b) 100°C
(c) 101°C
(d) 100.52°C
Answer:
(c) 101°C
Solution:
\(\frac { ΔP }{ P° }\) = \(\frac { { n }_{ 2 } }{ { n }_{ 1 } }\)
W2 = 6.5g
W1 = 100g
Kb = 0.52


Question 13.
According to Raoult’s law, the relative Lowering of vapour pressure for a solution is equal to…
(a) mole fraction of solvent
(b) mole fraction of solute
(c) number of moles of solute
(d) number of moles of solvent
Answer:
(b) mole fraction of solute
Solution:
\(\frac { ∆P }{ P° }\) = x2 (Mole fraction of the solute)
![]()
Question 14.
At same temperature. which pair of the following solutions are isotonic?
(a) 0.2 M BaCl2 and 0.2M urea
(b) 0.1 M glucose and 0.2 M urea
(c) 0.1 MNaCl and 0.1 MK2SO4
(d) 0.1 MBa(NO3)2 and 0.1 MNa2 SO4
Answer:
(d) 0.1 M Ba (NO3)2 and 0.1 M Na2 SO4
Solution:
0.1 x 3 ion [Ba2 + 2NO3–], 0.1 x 3 ion [2Na+, SO4–]
The formula of normality a solution is the gram equivalent weight of a solute per liter of solution.
Question 15.
The empirical formula of a non-electrolyte(X) is CH2O. A solution containing six gram of X exerts the same osmotic pressure as that of 0.025 M glucose solution at the same temperature. The molecular formula of X is
(a) C2H4O2
(b) C8H16O8
(c) C4H8O4
(d) CH2O
Answer:
(b) C8H16O8
Solution:
(π1)non electrolute = (π2)glucose
C1RT = C2RT
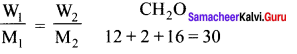
\(\frac { 6 }{ n(30) }\) = 0.025
n = \(\frac { 6 }{ 0.025 x 30 }\) = 30
∴ Molecular formula C8H16O8
Question 16.
The KH for the solution of oxygen dissolved in water is 4 x 104 atm at a given temperature. If the partial pressure of oxygen in air is 0.4 atm, the mole fraction of oxygen in solution is …………..
(a) 4.6 x 103
(b) 1.6 x 104
(c) 1 x 10-5
(d) 1 x 105
Answer:
(c) 1 x 10-5
Solution:
KH = 4 x 104 atm,
(PO2)air = 0.4 atm,
(xo2)in solution = ?
air – in solution
(PO2)air = KH(xo2)in solution
0.4 = 4 x 104(xo2)in solution
(xo2)in solution = \(\frac { 0.4 }{ 4\times { 10 }^{ 4 } }\) = 1 x 10-5
![]()
Question 17.
Normality of 1.25M sulphuric acid is …………
(a) 1.25 N
(b) 3.75 N
(c) 2.5 N
(d) 2.25 N
Answer:
(c) 2.5 N
Solution:
Normality of H2SO4 = (No. of replacable H+) x M = 2 x 1.25 = 2.5 N
Question 18.
Two liquids X and Y on mixing gives a warm solution. The solution is …………..
(a) ideal
(b) non-ideal and shows positive deviation from Raoults law
(c) ideal and shows negative deviation from Raoults Law
(d) non – ideal and shows negative deviation from Raoults Law
Answer:
(d) non – ideal and shows negative deviation from Raoults Law
Solution:
∆Hmix is negative and show negative deviation from Raoults law.
![]()
Question 19.
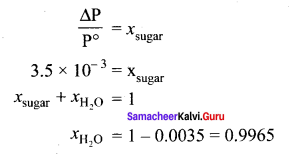
The relative lowering of vapour pressure of a sugar solution in water is 3.5 x 10-3. The mole fraction of water in that solution is …………
(a) 0.0035
(b) 0.35
(c) 0.0035/18
(d) 0.9965
Answer:
(d) 0.9965
Solution:
Question 20.
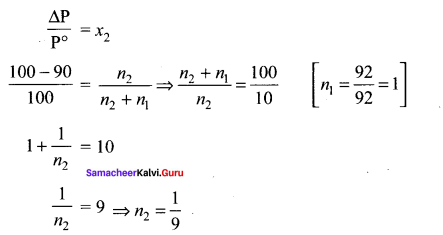
The mass of a non-volatile solute (molar mass 80 g mol-1) which should be dissolved in 92g of toluene to reduce its vapour pressure to 90% ………..
(a) 10g
(b) 20g
(c) 9.2 g
(d) 8.89g
Answer:
(d) 8.89g
Solution:
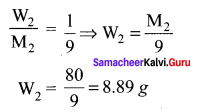
Question 21.
For a solution, the plot of osmotic pressure (π) verses the concentration (e in mol L-1) gives a straight line with slope 310 R where ‘R’ is the gas constant. The temperature at which osmotic pressure measured is ………..
(a) 310 x 0.082 K
(b) 3 10°C
(c) 37°C
(d) \(\frac { 310 }{ 20.082}\)
Answer:
(c) 37°C
Solution:
π = CRT
y = x(m)
m = RT
310 R = RT
T = 310 K
= 37°C
![]()
Question 22.
200 ml of an aqueous solution of a protein contains 1 .26g of protein. At 300K, the osmotic pressure of this solution is found to be 2.52 x 10-3 bar. The molar mass of protein will be (R =0.083 Lhar mol-1 K-1) ……………
(a) 62.22 Kg mol-1
(b) 12444 g mol-1
(c) 300g mol-1
(d) none of these
Answer:
(a) 62.22 Kg mol-1
Solution:
π = CRT
M = \(\frac { WRT }{ π1 }\) = \(\frac { 1.26\times 0.083\times 300 }{ 2.52\times { 10 }^{ -3 }\times 0.2 }\) = 62.22Kg mol-1
Question 23.
The Van’t Hoff factor (i) for a dilute aqueous solution of the strong electrolyte barium hydroxide is ………..
(a) 0
(b) 1
(c) 2
(d) 3
Answer:
(b) 1
Solution:
Ba(OH)2 dissociates to form Ba2+ and 2OH-1 ion
α = \(\frac { (i – 1) }{ (n – 1) }\)
i = α (n – 1) + 1
n = i = 3 ( for Ba (OH)2, α = 1 )
Question 24.
What is the molality of a 10% w/w aqueous sodium hydroxide solution?
(a) 2.778
(b) 2.5
(c) 10
(a) 0.4
Answer:
(b) 2.5
Solution:
100% \(\frac { w }{ w }\) aqueous NaOH solution means that 10 g of sodium hydroxide in 100g solution.
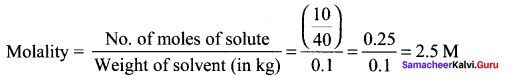
Question 25.
The correct equation for the degree of an associating solute, ‘n’ molecules of which undergoes association in solution, is ………
(a) α = \(\frac { n(i – 1) }{ n – 1 }\)
(b) α2 = \(\frac { n(1 – i) }{ n – 1 }\)
(c) α = \(\frac { n(i – 1) }{ 1 – n }\)
(d) α = \(\frac { n(1 – i) }{ n(1 – i) }\)
Answer:
(c) α = \(\frac { n(i – 1) }{ 1 – n }\)
Solution:
α = \(\frac { (i – 1)n }{ (n – 1) }\) (or) \(\frac { n(i – 1) }{ (1 – n) }\)
![]()
Question 26.
Which of the following aqueous solutions has the highest boiling point?
(a) 0.1 M KNO3
(b) 0.1 M Na3PO4
(c) 0.1 M BaCl2
(d) 0.1 M K2SO4
Answer:
(a) 0.1 M KNO3
Solution:
Elevation of boiling point is more in the case of Na3PO4(no. of ions 4; 3 Na+, PO43-)
Question 27.
The freezing point depression constant for water is 1.86° k kg mo1-1 . If 5g Na2SO4 is dissolved in 45g water, the depression in freezing point is 3.64°C. The van’t Hoff factor for Na2SO4 is ……..
(a) 2.50
(b) 2.63
(c) 3.64
(d) 5.50
Answer:
(a) 2.50
Solution:
Kf = 1.86
W2 = 5g
∆Tf = 3.64
M2 = 142
W1 = 45g
ΔTf = i x Kf

Question 28.
Equimolal aqueous solutions of NaCI and KCI are prepared. If the freezing point of NaCI is – 2°C, the freezing point of KCI solution is expected to be ………
(a) – 2°C
(b) – 4°C
(c) – 1°C
(d) 0°C
Answer:
(a) – 2°C
(b) – 4°C
(c) – 1°C
(d) 0°C
Solution:
Equimolal aqueous solution of KCI also shows 2° C depression in freezing point.
![]()
Question 29.
Phenol dimerises in henzene having van’t Hoff factor 0.54. What is the degree of association?
(a) 0.46
(b) 92
(c) 46
(d) 0.92
Answer:
(d) 0.92
Solution:
α = \(\frac { (1-i)n }{ (n-1) }\) = \(\frac { (1 – 0.54)2 }{ (2 – 1) }\) = 0.46 x 2 = 0.92
Question 30.
Assertion: An ideal solution obeys Raoult’s Law
Reason: In an ideal solution, solvent-solvent, as well as solute-solute interactions, are similar to solute-solvent interactions.
(a) both assertion and reason are true and reason is the correct explanation of assertion
(b) both assertion and reason are true but reason is not the correct explanation of assertion
(c) assertion is true but reason is false
(d) both assertion and reason are false
Answer:
(a) both assertion and reason are true and reason is the correct explanation of assertion
Samacheer Kalvi 11th Chemistry Solutions Short Answer Questions
Question 31.
Define
- Molality
- Normality
Answer:
1. Molality (m):
It is defined as the number of moles of the solute present in 1 kg of the solvent
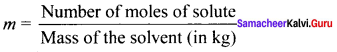
2. Normality (N):
It is defined as the number of gram equivalents of solute in I litre of the solution.
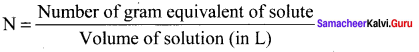
Question 32.
What is a vapour pressure of liquid? What is relative lowering of vapour pressure?
Answer:
1. The pressure of the vapour in equilibrium with its liquid ¡s called vapour pressure of the liquid at the given temperature.
2. The relative lowering of vapour pressure is defined as the ratio of lowering of vapour. pressure to vapour pressure of pure solvent. Relative lowering of vapour pressure
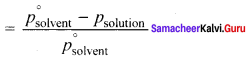
Question 33.
State and explain Henry’s law.
Answer:
“The partial pressure of the gas in vapor phase (vapour pressure of the solute) is directly proportional to the mole fraction (x) of the gaseous solute in the solution at low concentrations”. This statement is known as Henry’s law.
Henry’s law can be expressed as,
Psolute α xsolute in solution
Psolute = KH xsolute solution
Here, Psolute represents the partial pressure of the gas in vapour state which is commonly called vapour pressure. Xsolute in solution represents the mole fraction of solute in the solution. KH is an empirical constant with the dimensions of pressure.
![]()
Question 34.
State Raoult law and obtain expression for lowering of apour pressure when the nonvolatile solute is dissolved in solvent.
Answer:
Raoult’s law:
This law states that “in the case of a solution of volatile liquids the partial vapour pressure of each component (A & B) of the solution is directly proportional to its mole fraction.
PA ∝ x A
when xA = 1,
then k = P°A
(P°A = vapour pressure of pure component)
PA = P°A . xa
PB = P°B . xb
when a non-volatile is dissolved in pure water, the vapour pressure of the pure solvent will decrease. In such a solution, the vapours pressure of the solution will depend only on the solvent molecules as the solute is non-volatile.
Psolution ∝ xA
Psolution = k . xA
xA = 1, k = P°solvent
P°solution = P°solvent – Psolution
Lowering of vapour pressure = P°solvent – Psolution
Relative lowering of vapour pressure = \(\frac { P° – P }{ P° }\) = xB
where xB = Mole fraction of solute.
![]()
Question 35.
What is molal depression constant? Does it depend on nature of the solute?
Answer:
Kf = molar freezing point depression constant or cryoscopic constant.
∆Tf = Kf . m,
where
∆Tf = depression in freezing point.
m = molality of the solution
Kf = cryoscopic constant
If m = I
∆Tf = Kf
i.e., cryoscopic constant is equal to the depression in freezing point for 1 molal solution cryoscopic constant depends on the molar concentration of the solute particles. Kf is directly proportional to the molal concentration of the solute particles.
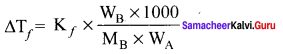
WB = mass of the solute
WA = mass of solvent
MB = molecular mass of the solute.
Question 36.
What is osmosis?
Answer:
“The phenomenon of the flow of solvent through a semipermeable membrane from pure solvent to the solution is called osmosis”. Osmosis can also be defined as “the excess pressure which must be applied to a solution to prevent the passage of solvent into it through the semipermeable membrane”. Osmotic pressure is the pressure applied to the solution to prevent osmosis.
Question 37.
Define the term isotonic
Answer:
1. Two solutions having same osmotic pressure at a given temperature are called isotonic solutions.
2. When such solutions arc separated by a semipermeable membrane, solvent flow between one to the other on either direction is same. i.e. the net solvent flow between two isotonic solutions is zero.
Samacheer Kalvi 11th Chemistry Solutions Long Answer Questions
Question 38.
You are provided with a solid ‘A’ and three solutions of A dissolved in water – one saturated, one unsaturated, and one supersaturated. How would you determine each solution?
Answer:
1. Saturated solution:
When the maximum amount of solute is dissolved in a solvent, any more addition of solute will result in precipitation at a given temperature and pressure. Such a solution is called a saturated solution.
2. Unsaturated solution:
When the minimum amount of solute is dissolved in a solvent at a given temperature and pressure is called an unsaturated solution.
3. Supersaturated solution:
It is a solution that holds more solute than it normally could in its saturated form.
Example:
- A saturated solution where the addition of more compound would not dissolve in the solution. 359 g of NaCI in 1 litre of water at 25°C.
- An unsaturated solution has the capacity to dissolve more of the compound. 36 g of NaCI in 1 litre of water at 25°C.
- A supersaturated solution is a solution in which crystals can start growing. 500 g of NaCI in 1 litre of water at 25°C.
![]()
Question 39.
Explain the effect of pressure on solubility.
Answer:
Generally, the change in pressure does not have any significant effect on the solubility of solids and liquids as they are not compressible. However, the solubility of gases generally increases with an increase in pressure.
Consider a saturated solution of a gaseous solute dissolved in a liquid solvent in a closed container. In such a system, the following equilibrium exists.
Gas (in a gaseous state) ⇌ Gas (in solution)
According to the Le-Chatelier principle, the increase in pressure will shift the equilibrium in the direction which will reduce the pressure. Therefore, more gaseous molecules dissolve in the solvent, and the solubility increases.
Question 40.
A sample of 12 M Concentrated hydrochloric acid has a density of 1.2 gL-1. Calculate the molality.
Answer:
Given:
Molarity = 12 M HCI
Density of the solution = 1.2 g L-1
In the 12 M HCl solution, there are 12 moles of HCl in 1 litre of the solution.
![]()
Calculate mass of water (solvent)
Mass of 1 litre HCI solution = density x volume
= 1.2gmL-1 x 1000 mL
= 1200g
Mass of LICI = No. of moles of HCI x molar mass of HCI
= 12mol x 36.5 g mol-1
= 438g
Mass of waler = mass of HCI solution – mass of HCI
Mass of waler = 1200 – 438 = 762 g
Molalily =\(\frac { 12 }{ 0.762 }\) = 15.75m
Question 41.
A 0.25 M glucose solution at 370.28 K has approximately the pressure as blood. What is the osmotic pressure of blood?
Solution.
C = 0.25 M
T = 370.28 K
(π)glucose = CRT
(π) = 0.25 mol L-1 × 0.082L atm K-1mol-1 × 370.28K
= 7.59 atm
Question 42.
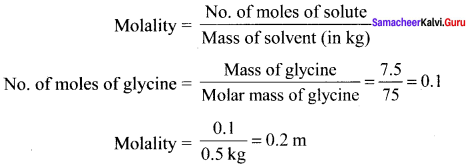
Calculate the molarity of a solution containing 7.5g of glycine (NH2 – CH2 – COOH) dissolved in 500g of water.
Solution:
Question 43.
Which solution has the lower freezing point? 10g of methanol (CH3OH) in 100g of water (or) 20g of ethanol (C2H5HO) In 200g of water.
Solution:
∆Tf = Kf . m i.e. ∆Tf ∝ m
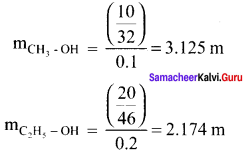
∴ Depression in freezing point is more in methanol solution and it will have a lower freezing point.
Question 44.
How many moles of solute particles are present in one litre of 10-4 M potassium sulphate?
Solution:
In 10-4M K2SO4 solution, there are 10-4 moles of potassium sulphate.
K2SO4 molecule contains 3 ions (2 K+ and 1SO42-)
1 mole of K2SO4 contains 3 x 6.023 x 1023 ions
10 mole of K2SO4 contains 3 x 6.023 x 102 x 10-4 ions = 18.069 x 1019
![]()
Question 45.
Henry’s law constant for solubility of methane in benzene is 4.2 x 10-5 mm Hg at a particular constant temperature. At this temperature, calculate the solubility of methane at
- 750 mm Hg
- 840 mmHg
Solution:
(KH)Benzene = 4.2 x 10-5 mm Hg. Solubility of methane = ? P = 750 mm Hg, p = 840 mm Hg
According to Henry’s Law,
P = KH . xsolution
750 mm Hg = 4.2 x 10-5 mm Hg . xsolution
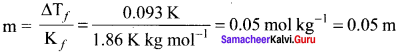
Question 46.
The observed depression in the freezing point of water for a particular solution is 0.093°C. Calculate the concentration of the solution in molality. Given that the molal depression constant for water is 1.86 K kg mol-1.
Solution:
T1= 0.093°C = 0.093K
m = ?
Kf = 1.86K kg mol-1
∆Tf = kf . m
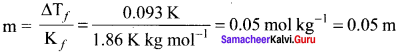
Question 47.
The vapour pressure of pure benzene (C6H6) at a given temperature is 640 mm Hg. 2.2 g of non-volatile solute is added to 40 g of benzene. The vapour pressure of the solution is 600 mm Hg. Calculate the molar mass of the solute?
Solution:
P0C6H6 = 640 mm Hg
W2 = 2.2 g (non volatile solute)
W1 = 40 g (benzene)
Psolution = 600 mm Hg
M2 = ?
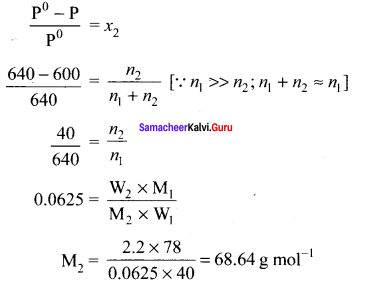
Samacheer Kalvi 11th Chemistry Solutions In Text Questions – Evaluate Yourself
Question 1.
If 5.6 g of KOH is present in (a) 500 mL and (b) I litre of solution, calculate the molarity of each of these solutions.
Solution.
Mass of KOH = 5.6g
No. of moles = \(\frac { 5.6 }{ 5.6 }\) = 0.1 mol
1. Volume of the solution = 500 ml = 0.5 L
2. Volume of the solution = IL
![]()
3. Volume of the solution = IL
Molarity = \(\frac { 0.1 }{ 1 }\) M
Question 2.
2.82 g of glucose is dissolved in 30 g of water. Calculate the mole fraction of glucose and water.
Solution:
Mass of glucose = 2.82 g
No. of moles of glucose = \(\frac { 2.82 }{ 180 }\) = 0.0 16
Mass of water = 30g = \(\frac { 30 }{ 18 }\) = 1.67
xH2O = \(\frac { 1.67 }{ 1.67 + 0.016 }\) = \(\frac { 1.67 }{ 1.686 }\) = 0.99
xH2O + xglucose = 1
0.99 + xglucose = 1
xglucose = 1 – 0.99 = 0.01
![]()
Question 3.
The antiseptic solution of iodopovidone for the use of external application contains 10% w/v of iodopovidone. Calculate the amount of iodopovidone present in a typical dose of 1.5 mL.
Solution:
10% \(\frac { w }{ v }\) means that 10 g of solute in 100 ml solution
∴ Amount of iodopovidone in 1.5 ml = \(\frac { 10g }{ 100ml }\) x 1.5 ml = 0.15 g
Question 4.
A litre of sea water weighing about 1.05 kg contains 5 mg of dissohed oxygen (O2). Express the concentration of dissolved oxygen in ppm.
Solution:

Question 5.
Describe how would you prepare the following solution from pure solute and solvent
- 1 L of aqueous solution of 1.5 M COCI2.
- 500 mL of 6.0 % (v/v) aqueous methanol solution.
Solution:
- mass of 1.5 moles of COCI2 = 1.5 x 129.9 = 194.85g
- 194.85g anhydrous cobalt chloride is dissolved in water and the solution is make up to one litre in a standard flask.
Question 6.
How much volume of 6 M solution of NaOH is required to prepare 500 mL of 0.250 M NaOH solution.
Solution:
6% \(\frac { v }{ v }\) aqueous solution contains 6g of methanol in 100 ml solution. To prepare 500 ml of 6% v/v solution of methanol 30g methanol is taken in a 500 ml standard flask and required quantity of water is added to make up the solution to 500 ml.
Question 7.
Calculate the proportion of O2 and N2 dissolved in water at 298 K. When air containing 20% O2 and 80% N2 by volume is in equilibrium with water at 1 atm pressure. Henry’s law constants for two gases are KH(O2) = 4.6 x atm and KH (N2) 8.5 x 104 atm.
Solution:
C1V1 = C2V2
6M (V1) = 0.25M x 500 ml
V1 = \(\frac { 0.25 x 500 }{ 6 }\)
V1 = 20.3 mL
![]()
Question 8.
Explain why the aquatic species are more comfortable in cold water during winter season rather than warm water during the summer.
Solution:
Total pressure = 1 atm
PN2 = \((\frac { 80 }{ 100 })\) x Total pressure = \(\frac { 80 }{ 100 }\) x 1 atm = 0.8 atm
PO2 = \((\frac { 20 }{ 100 })\) x 1 = 0.2 atm
According to Henry’s Law
Psolute = KH x solute in solution
PN2 = (KH)Nitrogen x Mole fraction of Nitrogen in solution

Question 9.
Calculate the mole fractions of benzene and naphthalene in the vapour phase when an ideal liquid solution is formed by mixing 128 g of naphthalene with 39g of benzene. It is given that the vapour pressure of pure benzene is 50.71 mm Hg and the vapour pressure of pure naphthalene is 32.06 mm Hg at 300 K.
Solution:
P0pure benzene = 50.71 mm Hg
P0nepthalene = 32.06 mm Hg
Number of moles of benzene = \(\frac { 39 }{ 78 }\) = 0.5 mol
Number of moles of naphthalcne = \(\frac { 128 }{ 128 }\) =1 mol
Mole fraction of benzene = \(\frac { 0.5 }{ 1.5 }\) = 0.33
Mole fraction of naphthalene = 1 – 0.33 = 0.67
Partial vapour pressure of benzene =P0benzene x Mole fraction of benzene
= 50.71 x 0.33 = 16.73 mm Hg
Partial vapour pressure of naphthalene = 32.06 x 0.67 = 21.48mm Hg
Mole fraction of benzene in vapour phase = \(\frac { 16.73 }{ 16.73 + 21.48 }\) = \(\frac { 16.73 }{ 38.21 }\) = 0.44
Mole fraction of naphthalene in vapour phase = 1 – 0.44 = 0.56
![]()
Question 10.
Vapour pressure of a pure liquid A is 10.0 torr at 27°C. The vapour pressure is lowered to 9.0 torr on dissolving one grani of B in 20g of A. If the molar mass of A is 200 g mol-1 then calculate the molar mass of B.
Solution:
P0A = 10 torr
Psolution = 9 torr
WA = 20 g
WB = 1 g
MA = 200 g mol-1
MB = ?
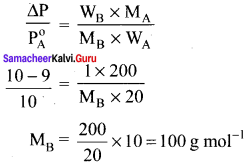
Question 11.
2.56g of Sulphur is dissolved in 100g of carbon disuiphide. The solution boils at 319.692K. What is the molecular formula ofSulphur in solution? The boiling pointof CS2 is 319. 450K. Given that Kb for CS2 = 2.42 K kg mol-1
Solution:
W2 = 2.56g
W1 = 100g
T = 319.692 K
Kb = 2.42 K kg mol-1
∆Tb = (319.692 – 319.450) K = 0.242 K
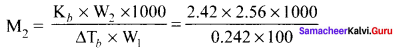
M2 = 256g mol-1
Molecular mass of sulphur in solulion = 256 g mol-1
Atomic mass of one mole of sulphur atom = 32
No. of atoms in a molecule of sulphur = \(\frac { 256 }{ 32 }\) = 8
Hence, molecular tòrmula of sulphur is S8.
Question 12.
2g of a non-electrolyte solute dissolved in 75g of benzene lowered the freezing point of benzene by 0.20 K. The freezing point depression constant of benzene is 5.12 K Kg mol-1. Find the molar mass of the solute.
Solution:
W2 = 2g
W1 = 75g
∆Tf = 0.2 K
kf = 5.12 K kg mol-1
M2 = ?
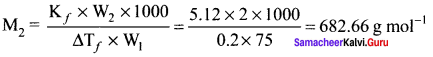
Question 13.
What is the mass of glucose (C6H12O6) in its one-litre solution is isotonic with 6g L-1 of urea (NH2CONH2)?
Solution:
The osmotic pressure of urea solution (π1) = CRT
\(\frac { { W }_{ 2 } }{ { M }_{ 2 }V }\)RT = \(\frac { 6 }{ 60 x 1 }\) x RT
The osmotic pressure of glucose solution
(π2) \(\frac { { W }_{ 2 } }{ 180\times 1 }\) x RT
For isotonic solution, π1 = π2
\(\frac { 6 }{ 60 }\) = \(\frac { { W }_{ 2 } }{ 180\times 1 }\) RT
⇒ W2 = \(\frac { 6 }{ 60 }\) x 180
⇒ W2 = 18 g
Question 14.
0.2m aqueous solution of KCI freezes at – 0.68°C calculate van’t Hoff factor. Kf for water is 1.86 K kg mol-1.
Solution:

Given,
∆Tf = 0.680 K
m = 0.2 m,
∆Tf (observed) = 0.680K
∆Tf (Calculated) = kf
m = 1.86 K kg mol-1 x 0.2 mol kg-1 = 0.372K
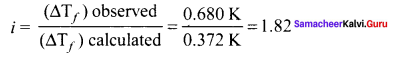
Samacheer Kalvi 11th Chemistry Solutions Example problems Solved
Question 1.
What volume of 4M HCI and 2M HCI should be mixed to get 500 mL of 2.SM HCI?
Solution:
Let the volume of 4M HCl required to prepare 500 mL of 2.5 M HCI = x mL
Therefore, the required volume of 2M HCI = (500 – x) mL
We know from the equation x = \(\frac { 250 }{ 2 }\) = 125 mL
Hence, volume of 4M HCI required = 125 mL
Volume of 2M HCl required = (500 – 125) mL = 375 mL
Question 2.
0.24g of a gas dissolves in 1 L of water at 1.5 atm pressure. Calculate the amount of dissolved gas when the pressure is raised to 6.0 atm at constant temperature.
Solution:
Psolute = KH xsolute in solution
At pressure 1.5 atm, p1 = KH x1 ………..(1)
At pressure 6.0 atm, p2 = KHx2 …………..(2)
Dividing equation (1) by (2)
Weget
\(\frac { { P }_{ 1 } }{ { P }_{ 2 } }\) = \(\frac { { x }_{ 1 } }{ { x }_{ 2 } }\)
\(\frac { 1.5 }{ 6.0 }\) = \(\frac { { 0.24 } }{ { x }_{ 2 } }\)
Therefore
x2 = \(\frac { 0.24×6.0 }{ 1.5 }\) = 0.96 g/L
![]()
Question 3.
An aqueous solution of 2% nonvolatile solute exerts a pressure of 1.004 bar at the boiling point of the solvent. What is the molar mass of the solute when PA° is 1.013 bar?
Solution:
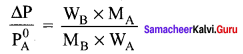
In a 2% solution weight of the solute is 2g and solvent is 98g
ΔP = PA0 – Psolution = 1.013 – 1.004 bar = 0.009 bar

Question 4.
0.75 g of an unknown substance is dissolved in 200 g solvent. If the elevation of boiling point is 0.15 K and molal elevation constant is 7.5K kg more then, calculate the molar mass of unknown substance.
Solution:
∆Tb = Kb m = Kb x W2 x 1000/M2 x W1
M2 = Kb x W2 x 1000/∆Tb x W1
= 7.5 x 0.75 x 1000/0.15 x 200 = 187.5g mol-1
Question 5.
Ethylene glycol (C2H6O2) can be used as an antifreeze in the radiator of a car. Calculate the temperature when ice will begin to separate from a mixture with 20 mass percent of glycol in water used in the car radiator. Kf for water = 1.86 K kg mol-1 and molar mass of ethylene glycol is 62g mol-1.
Solution:
Weight of solute (W2) = 20 mass percent of solution means 20g of ethylene glycol
Weight of solvent (water) W1 = 100 – 20 = 80g
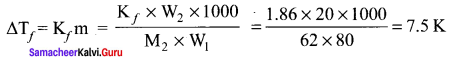
The temperature at which the ice will begin to separate is the freezing of water after the addition of solute i.e. 7.5 K lower than the normal freezing point of water (273 – 7.5)K = 265.5K
Question 6.
At 400K 1.5 g of an unknown substance is dissolved in solvent and the solution is made to 1.5 L. Its osmotic pressure is found to be 0.3 bar. Calculate the molar mass of the unknown substance.
Solution:

Question 7.
The depression in freezing point is 0.24K obtained by dissolving 1g NaCI in 200g water. Calculate van’t – Hoff factor. The molal depression constant is 1.86 K kg mol-1.
Solution:
Sol. Molar mass of solute
Samacheer Kalvi 11th Chemistry Solutions Additional Questions Solved
Samacheer Kalvi 11th Chemistry Solutions 1 Mark Questions and Answers
I. Choose the correct answer.
Question 1.
Among the following, which one is mostly present in seawater?
(a) NaCI
(b) Nal
(c) KCI
(d) MgBr2
Answer:
(a) NaCI
Question 2.
Statement I: The most common property of seawater and air is homogeneity.
Statement II: The homogeneity implies uniform distribution of their constituents through the mixture.
(a) Statements I and II arc correct and II are the correct explanation of I.
(b) Statements I and II are correct but II is not the correct explanation of I.
(c) Statement I is correct but II is wrong.
(d) Statement I is wrong but II is correct.
Answer:
(a) statement I and II are correct and II is the correct explanation I.
Question 3.
Which one of the following is a homogeneous mixture?
(a) Seawater
(b) Air
(c) Alloys
(d) All the above
Answer:
(d) All the above
![]()
Question 4.
Statement I: The salt solution is an aqueous solution.
Statement II: If water is used as the solvent, the resultant solution is called an aqueous solution.
(a) Statements I and II are correct but II is not the correct explanation of I.
(b) Statements I and II are correct and II is the correct explanation of I.
(c) Statement I is correct but statement II is wrong.
(d) Statement I is wrong but statement II is correct.
Answer:
(b) Statements I and II are correct and II is the correct explanation of I.
Question 5.
Statement I: The dissolution of ammonium nitrate increases steeply with an increase in temperature.
Statement II: The dissolution process of ammonium nitrate is endothermic in nature.
(a) Statement I and II are correct and statement II is the correct explanation of statement I.
(b) Statement I and II are correct but II is not the correct explanation of I.
(c) Statement I is correct but II is wrong.
(d) Statement I is wrong but II is correct.
Answer:
(a) Statement I and II are correct and statement II is the correct explanation of statement I.
Question 6.
In which of the following compound the solubility decreases with the increase of temperature?
(a) sodium chloride
(b) ammonium nitrate
(c) ceric sulphate
(d) calcium chloride
Answer:
(c) ceric sulphate
Question 7.
Which of the following is not an ideal solution?
(a) Benzene & toluene
(b) n – Hexane & n – Heptane
(c) Ethyliodide & ethyl bromide
(d) Ethanol and water
Answer:
(d) Ethanol and water
Question 8.
Which one of the following shows positive deviation from Raoult’s law?
(a) Ethyliodide and Ethyl bromide
(b) Ethyl alcohol and cyclohexane
(c) Chioro benzene & bromo benzene
(d) Benzene & toluenc
Answer:
(b) Ethyl alcohol and cyclohexane
Question 9.
Which one of the following is not a non-ideal solution showing positive deviation?
(a) Benzene & acetone
(b) CCl4 & CHCl3
(c) Acetone & ethyl alcohol
(d) Benzene and toluene
Answer:
(d) Benzene and toluene
![]()
Question 10.
Which of the following shows a negative deviation from Raoult’s law?
(a) Phenol and aniline
(b) Benzene and toluene
(c) Acetone and ethanol
(d) Bcnzene and acetone
Answer:
(a) Phenol and aniline
Question 11.
Which of the following is not a non-ideal solution showing negative deviation?
(a) Phenol and aniline
(b) Ethanol and water
(c) Acetone + Chloroform
(d) n – Heptane and n – Hexane
Answer:
(d) n – Heptane and n-Hexane
Question 12.
Statement I: A solution of potassium chloride in water deviates from ideal behavior.
Statement II: The solute dissociates to give K and Cl ions which form strong ion-dipole interaction with water molecules.
(a) Statement I & II are correct and II is the correct explanation of I
(b) Statement I & II are correct but II is not the correct explanation of I
(c) Statement I is correct but statement II is wrong.
(d) Statement I is wrong but statement II is correct.
Answer:
(a) Statement I & II are correct and II is the correct explanation of I
Question 13.
Statement I: Acetic acid deviates from ideal behavior.
Statement II: Acetic acid exists as a dimer by forming intermolecular hence deviates from Raoult’s law.
(a) Statement I & II are correct and II is the correct explanation of I.
(b) Statement I & II are correct but II is not the correct explanation of I.
(c) Statement I is true but II is wrong.
(d) Statement I is wrong but II is correct.
Answer:
(a) Statement I & II are correct but II is the correct explanation of I.
![]()
Question 14.
Which one of the following has found to have abnormal molar mass? hydrogen bonds and
(a) NaCl
(b) KCI
(c) Acetic acid
(d) all the above
Answer:
(d) All the above
Question 15.
What would be the value of the van’t Hoff factor for a dilute solution of K2SO4 in water?
(a) 3
(b) 2
(c) 1
(d) 4
Answer:
(a) 3
Solution:
ions produced = n = 3
Since
K2SO4 → 2K+ + SO42-
K2SO4 is completely dissociated so
∝ = \(\frac { i – 1 }{ n – 1 }\) = \(\frac { i – 1 }{ 3 – 1 }\) = 1
i – 1 = 1 x 2
i – 1 = 2
i = 2+1 = 3
Question 16.
In the determination of the molar mass of AB using a colligative property, what may be the value of van’t Hoff factor if the solute is 50% dissociates?
(a) 0.5
(b) 1.5
(c) 2.5
(d) 1
Answer:
(b) 1.5
Solution:
∝ = \(\frac { i – 1 }{ n – 1 }\) = 0.5
\(\frac { i – 1 }{ 2 – 1 }\) = 0.5
i – 1 = 0.5
i = 0.5 + 1 = 1.5
Question 17.
Which of the following solution has the highest boiling point?
(a) 5.85% solution of NaCI
(b) 18.0% solution of glucose
(c) 6.0% solution of urea
(d) All have the same boiling point
Answer:
(a) 5.85% solution of NaCl
Question 18.
Which one of the following pair is called an ideal solution?
(a) nicotine – water
(b) water – ether
(c) water – alcohol
(d) Chiorobenzene – bromobenzene
Answer:
(d) Chiorobenzene – bromobenzene
![]()
Question 19.
Which of the following is not a colligative property?
(a) optical activity
(b) osmotic pressure
(c) elevation boiling point
(d) depression in freezing point
Answer:
(a) optical activity
Question 20.
On dissolving sugar in water at room temperature solution feels cool to touch. Under which of the following cases dissolution of sugar will be most rapid?
(a) Sugar crystals in cold water
(b) Sugar crystals in hot water
(c) powdered sugar in cold water
(d) powdered sugar in hot water
Answer:
(d) powdered sugar in hot water
II. Match the following.
Question 1.
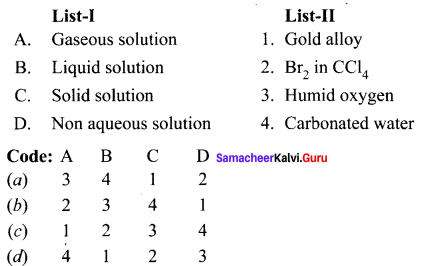
Answer:
(a) 3 4 1 2
Question 2.
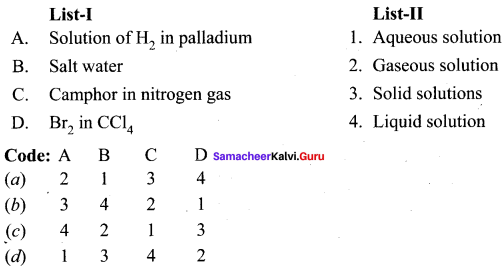
Answer:
(d) 3 4 2 1
Question 3.
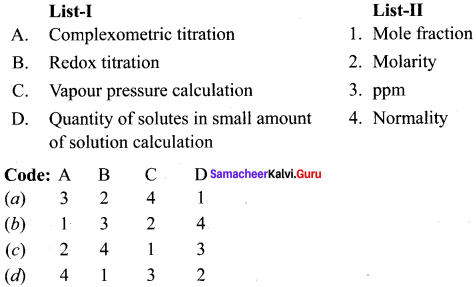
Answer:
(c) 2 4 1 3
Question 4.
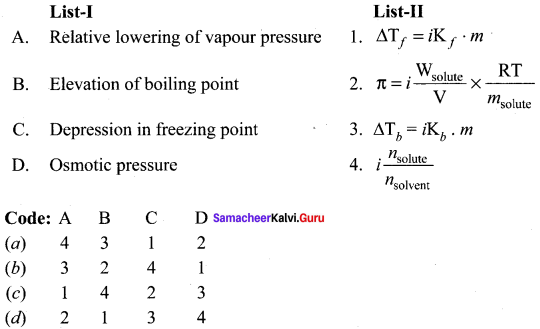
Answer:
(a) 4 3 1 2
Question 5.
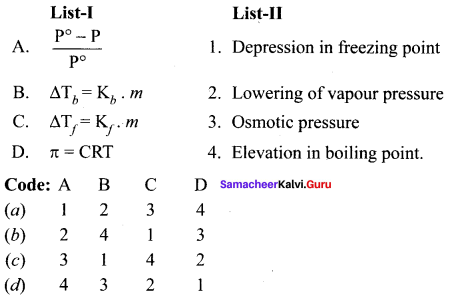
Answer:
(b) 2 4 1 3
III. Fill in the blanks.
Question 1.
……… covers more than 70% of the earth’s surface.
Answer:
Seawater
Question 2.
……… is an important naturally occurring solution.
Answer:
Air
Question 3.
An example of solid homogeneous mixture is ……….
Answer:
Brass
Question 4.
A mixture of N2, O2, CO2 and other traces of gases is known as ………
Answer:
Air
Question 5
……… a non – aqueous solution.
Answer:
Br2 in CCl4
Question 6.
……… is an example of a gaseous solution.
Answer:
Camphor in nitrogen gas
Question 7.
……… is used for a dental filling.
Answer:
Amalgam of potassium
![]()
Question 8.
Carbonated water is an example for ………
Answer:
Liquid solution
Question 9.
Humid oxygen is an example of ………
Answer:
Gaseous solution
Question 10.
The concentration of commercially available H2O2 is ………
Answer:
3%
Question 11.
The molality of the solution containing 45g of glucose dissolved in 2kg of water is ………
Answer:
0.125m

Question 12.
5.845 g of NaCl is dissolved in water and the solution was made up to 500 mL using a standard flask. The strength of the solution in molarity is ………
Answer:
0.2 M
Solution:
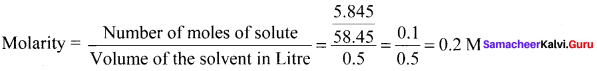
Question 13.
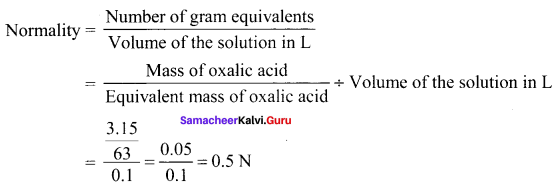
3.15 g of oxalic acid dihydrate is dissolved in water and the solution was made up to 100 ml using a standard flask. The strength of the solution in normality is ………
Answer:
0.5N
Solution:
Question 14.
5.85 g of NaCl is dissolved in water and the solution was made upto 500 ml using a standard flask. The strength of the solution in formality is ………
Answer:
0.2 F
Solution:
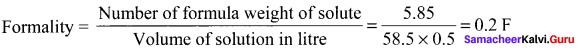
Question 15.
Neomycin, aminoglycoside antibiotic cream contains 300 mg of neomycin sulphate the active ingredient in 30 g of ointment base. The mass percentage of neomycin is ………
Answer:
1%
Solution:
The mass percentage of neomycin
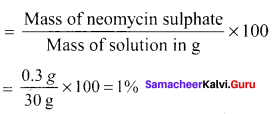
Question 16.
0.5 mole of ethanol is mixed with 1.5 moles of water. Then the mole fraction of ethanol and water are ……….
Answer:
0.25, 0.75
Solution:
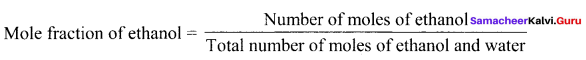
= \(\frac { 0.5 }{ 1.5 + 0.5 }\) = \(\frac { 0.5 }{ 2.0 }\) = 0.25
Mole fraction of water = \(\frac { 1.5 }{ 2.0 }\) = 0.75
Question 17.
50 mL of tincture of benzoin, an antiseptic solution contains 10 ml of benzoin. The volume percentage of benzoin is ……….
Answer:
20%
Solution:
Volume percentage of benzoin

= \(\frac { 10 }{ 50 }\) x 100 = 20%
Question 18.
A 60 ml of paracetamol pediatric oral suspension contains 3g of paracetamol. The mass percentage of paracetamol is …………
Answer:
5%
Solution:
Mass percentage of paracetamol =
![]()
= \(\frac { 3 }{ 60 }\) x 100 = 5%
Question 19.
50 ml of tap water contains 20 mg of dissolved solids. The TDS value in ppm is ………..
Answer:
400 ppm
Solution:
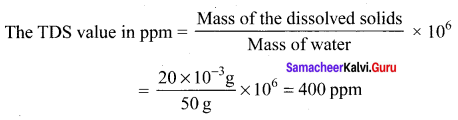
Question 20.
The concentration term used in the neutralisation reactions is …………
Answer:
Normality
Question 21.
The concentration term is used in the calculation of vapour pressure of the solution is …………..
Answer:
Mole fraction
Question 22.
The term used to express the active ingredients present in therapeutics is ………
Answer:
Percentage units
Question 23.
When the maximum amount of solute is dissolved in a solvent at a given temperature, the solution is called ………..
Answer:
Saturated solution
![]()
Question 24.
The solvent in which sodium chloride readily dissolves is …………
Answer:
Water
Question 25.
………… is used by deep-sea divers.
Answer:
Helium, nitrogen and oxygen
Question 26.
The mathematical expression of Raoult’s law is ………..
Answer:
PA = PA0 . XA
Question 27.
……….. is an ideal solution?
Answer:
Chloro benzene & bromo benzene
Question 28.
………….. is important in some vital biological systems.
Answer:
osmotic pressure
Question 29.
………. is not a colligative property.
Answer:
vapour pressure
Question 30.
According to van’t Hoff equation. the value of osmotic pressure t is equal to …………
Answer:
π = CRT
Question 31.
The osmotic pressure of the blood cells is approximately equal to 37°C.
Answer:
7 atm.
Question 32.
Which one of the following is applied in water purification?
Answer:
reverse osmosis
![]()
Question 33.
In the commercial reverse osmosis process, the semi-permeable membrane used is ………..
Answer:
cellulose acetate
Question 34.
The degree of dissociation α is equal to ……….
Answer:
\(\frac { i – 1 }{ n – 1 }\)
Question 35.
The degree of association a is equal to ……….
Answer:
\(\frac { (i – 1)n }{ n – 1 }\)
Question 36.
The estimated vantt Hoff factor for acetic acid solution in benzene is ………..
Answer:
0.5
Question 37.
The estimated van’t Hoff factor for sodium chloride in water is ………..
Answer:
2
Question 38.
Number of moles of the solute dissolved per dm3 of solution is ……….
Answer:
molarity
Question 39.
Molarity of pure water is ………….
Answer:
55.55
Solution:

Question 40.
18 g of glucose is dissolved in 90 g of water. The relative lowering of vapour pressure is equal to ………..
Answer:
0.1
Solution:
\(\frac { P° – P }{ P° }\) = x2
x2 = No. of moles of glucose
\(\frac { 18 }{ 180 }\) = 0.1
\(\frac { P° – P }{ P° }\) = 0.1
Question 41.
When NaCl is dissolved in water, boiling point ………..
Answer:
increases
Question 42.
Use of glycol as antifreeze in an automobile is an important application of …………….
Answer:
Colligative property
![]()
Question 43.
Ethylene glycol is mixed with water and used as antifreeze in radiators because …………..
Answer:
it lowers the freezing point of water
Question 44.
The colligative properties of a solution depend on ………… present in it.
Answer:
Number of solute particles
Question 45.
Low concentration of oxygen in the blood and tissues of people living at high altitude is due to ………….
Answer:
low atmospheric pressure
IV. Choose the odd one out.
Question 1.
(a) Air
(b) Camphor in nitrogen gas
(c) Humid oxygen
(d) Saltwater
Answer:
(d) Saltwater.
a, b and e are gaseous solutions whereas d is a liquid solution.
Question 2.
(a) CO2 dissolve in water
(b) Saltwater
(c) Solution of H2 in palladium
(d) Ethanol dissolved in water
Answer:
(c) Solution of H2 in palladium
a, b and d are liquid solutions whereas c is a solid solution.
Question 3.
(a) Amalgam of potassium
(b) Camphor in nitrogen gas
(c) Solution of H2 in palladium
(d) Gold alloy
Answer:
(b) Camphor in nitrogen gas
a, b and d arc solid solutions whereas b is the gaseous solution.
![]()
Question 4.
(a) Vapour pressure
(b) Lowering of vapour pressure
(c) Osmotic pressure
(d) Elevation of boiling point
Answer:
(a) Vapour pressure
b, e and dare colligative properties whereas a is a physical property.
Question 5.
(a) Benzene and tolucne
(b) Chlorobenzene and Bromobenzene
(c) Benzene and acetone
(d) n – hexane and n – heptane
Answer:
(a) Benzene and acetone
a, b, and d are ideal solutions whereas c is a non-ideal solution.
Question 6.
(a) Ethyl alcohol and cyclohexane
(b) Ethyl bromide and ethyl iodide
(c) Acetone and ethyl alcohol
(d) Benzene and acetone
Answer:
(a) Ethyl bromide and ethyl iodide
a, e and dare non-ideal solutions whereas b is an ideal solution.
V. Choose the correct pair.
Question 1.
(a) Humid oxygen – Liquid solution
(b) Gold alloy – Solid solution
(c) Saltwater – Gaseous solution
(d) Solution of H2 in palladium – Gaseous solution
Answer:
(b) Gold alloy – Solid solution
Question 2.
(a) Air – Gaseous solution
(b) Amalgam of potassium – Liquid solution
(c) Saltwater – Solid solution
(d) Carbonated water – Solid solution
Answer:
(a) Air – Gaseous solution
![]()
Question 3.
(a) Benzene and toluene – Non-ideal solution
(b) Benzcnc and acetone – Non-ideal solution
(c) Chlorobenzene and bromo henzene – Non-ideal solution
(d) Carbon tetrachloride and Chloroform – the ideal solution
Answer:
(b) Benzene and acetone – Non-ideal solution
Question 4.
(a) Benzene and toluene – Ideal solution
(b) n-hexane and n-heptane – Non-ideal solution
(c) Ethyl iodide and ethyl bromide – Non-ideal solution
(d) Chiorobenzene and bromo benzene – Non-ideal solution
Answer:
(a) Benzene and toluene – Ideal solution
VI. Choose the incorrect pair.
Question 1.
Answer:
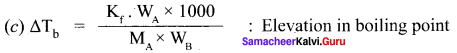
Question 2.
(a) Benzene and acetone – Ideal solution
(b) Ethyl alcohol and cyclohexane – Non-ideal solution
(C) n-hexane and n-heptane – Ideal solution
(d) Chioro benzene – Ideal solution
Answer:
(a) Benzene and acetone – Ideal solution
VII Assertion & Reason.
Question 1.
Assertion (A): When NaCI is added to water, depression in the freezing point is observed.
Reason (R): The lowering of vapour pressure of a solution causes the depression in freezing point.
(a) Assertion and Reason are correct and R is the correct explanation of A.
(b) Both A and R are correct but R is not the correct explanation of A.
(c) A is correct but R is wrong
(d) A is wrong but R is correct
Answer:
(a) Assertion and Reason are correct and R is the correct explanation of A.
![]()
Question 2.
Assertion (A): Ammonia reacts with water does not obey Henry’s law.
Reason (R): The gases reacting with the solvent does not obey Henry’s law.
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
(b) Both (A) and (R) are correct but (R) is not the correct explanation of (A).
(c) (A) is correct hut (R) is wrong.
(d) (A) is wrong but (R) is correct.
Answer:
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A).
Question 3.
Assertion (A): Acetic acid solution deviates from Raoult’s law.
Reason (R): Association of solute molecules exists as a dimer by forming intermolecular. hydrogen bonds and hence deviates from Raoult’s law.
(a) Both (A) and (R) arc wrong.
(b) Both (A) and (R) are correct and (R) is the correct explanation of (A).
(c) (A) is correct but (R) is wrong.
(d) (A) is wrong but (R) is correct.
Answer:
(b) Both (A) and (R) are correct and (R) is the correct explanation of (A).
VIII. Choose the correct statement.
Question 1.
(a) Raoult’s law is applicable to volatile solid solute in liquid solvent
(b) Henry’s law is applicable to solution containing solid solute in liquid solvent
(c) For very dilute solutions, the solvent obeys Raoult’s law and the solute obeys Henry’s law.
(d) For saturated solution containing volatile solid solute in liquid solvent both laws are obeyed.
Answer:
(c) For very dilute solutions. the solvent obeys Raoult’s law and the solute obeys LIenrys law.
Samacheer Kalvi 11th Chemistry Solutions 2 Marks Questions and Answers
I. Write brief answer to the following questions:
Question 1.
Define solution.
Answer:
A solution is a homogeneous mixture of two or more substances, consisting of atoms, ions or molecules. The constituent of the homogeneous mixture present in a lower amount is called the solute, and the one present in a larger amount is called the solvent. For example, when a small amount of NaCl dissolved in water.
![]()
Question 2.
Define the solution with an example.
Answer:
1. A solution is a homogeneous mixture of two or more substances consisting of atoms. ions or molecules.
2. For example, when a small amount of NaCl is dissolved in water, a homogeneous solution is obtained. In this solution, Na+ and C– ions are uniformly distributed in the water. Here NaCI is the solute and water is the solvent.
Question 3.
What is an unsaturated solution?
Answer:
An unsaturated solution is one that contains less amount of solute than its capacity to dissolve.
Question 4.
Define molality.
Answer:
Molality is defined as the number of moles of solute present in 1 kg of the solvent.

Question 5.
Define molarity.
Answer:
Molarity is defined as the number of moles of solute present in 1 litre of the solution.
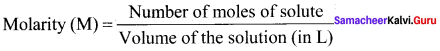
Question 6.
Define normality.
Answer:
Normality is defined as the number of gram equivalents of solute present in 1 litre of the solution.
![]()
Question 7.
Define formality.
Answer:
Formality (F) is defined as the number of formula weight of solute present in 1 litre of the solution.
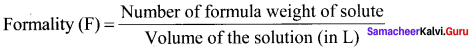
Question 8.
Define mole fraction.
Answer:
The mole fraction of a component is the ratio of a number of moles of the component to the total number of moles of all components present in the solution.
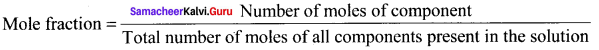
Question 9.
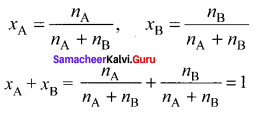
Show that the sum of mole fraction of a solution is equal to one.
Answer:
Consider a solution containing two components A and 13 whose mole fractions are xA and xB respectively. Let the number of moles of two components A and B are nA and nB respectively.
Question 10.
Define mass percentage.
Answer:
Mass percentage is defined as the ratio of the mass of the solute in g to the mass of solution in g multiplied by 100.

Question 11.
Define volume percentage.
Answer:
Volume percentage is defined as the ratio of volume of solute in mL to the volume of solution in ml multiplied by 100.

Question 12.
Define mass by volume percentage.
Answer:
It is defined as the ratio of the mass of the solute in g to the volume of the solution in ml multiplied by 100.
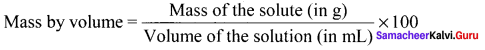
Question 13.
What is meant by ppm? Where is it used?
Answer:
1. part per million =
![]()
2. ppm is used to express the quantity of solutes present in small amounts in solutions.
Question 14.
What is the elevation of boiling point? Give it.
Answer:
The temperature difference between the solution and pure solvent is called elevation of boiling point,
∆Tb = T – T°
Unit of ∆Tb is K Kg mole-1.
![]()
Question 15.
Define solubility.
Answer:
The solubility of a substance is defined as the amount of the solute that can be dissolved in loo g of the solvent at a given temperature to form a saturated solution.
Question 16.
Ammonia is more soluble than oxygen in water. Why?
Answer:
Ammonia forms hydrogen bonding with water molecules, this intermolecular bonds arc very strong and thus the ammonia is more soluble in water. Ammonia is strongly interact with water to form ammonium hydroxide. But oxygen is more electronegative it is not able to interact with water more. So NH3 is more soluble than O2 in water.
Question 17.
Solubility of a solid solute in a liquid solvent increases with increase in temperature. Justify this statement.
Answer:
When the temperature is increased,the average kinetic energy of the molecules of the solute and the solvent increases. The increase in the kinetic energy facilitates the solvent molecules to break the intermolecular attractive forces that keep the solute molecules together and hence the solubility increases.
Question 18.
Dissolution of ammonium nitrate increases with increase in temperature. Why?
Answer:
The dissolution process of ammonium nitrate is endothermic. So the solubility increases with increase in temperature.
Question 19.
What is the relationship between the solubility of eerie sulphate with temperature?
Answer:
The dissolution of eerie sulphate is exothermic and the solubility decreases with the increase in temperature.
Question 20.
Why in the dissolution of CaCl2, the solubilit increases moderately with high temperature?
Answer:
Even though the dissolution of CaCI2, is cxothcrmic, the soluhility increases moderately with increase in temperature. Here the entropy factor plays a significant role in deciding the position of equilibrium.
Question 21.
Why the carbonated drinks are stored in pressurized container?
Answer:
1. The carbonated beverages contain CO2 dissolved in them. To dissolve the CO2 in these drinks, CO2 gas is bubbled through them under high pressure.
2. These containers are sealed to maintain the pressure. When we open these containers at atmospheric pressure, the pressure of the CO2 drops to the atmospheric pressure level and hence bubbles of CO2 rapidly escape from the solution and show effervescence.
![]()
Question 22.
Define
- Evaporation
- Condensation.
Answer:
1. Evaporation:
If the kinetic energy of molecules in the liquid state overcomes the intermolecular force of attraction between them, then the molecules will escape from the liquid state. This process is called evaporation.
2. Condensation:
The vapour molecules are in random motion during which they collide with each other and also with the walls of the container. As the collision is inelastic, they lose their energy and as a result, the vapour returns back to a liquid state. This process is called condensation.
Question 23.
State Dalton’s law of partial pressure.
Answer:
According to Dalton’s law of partial pressure, the total pressure in a closed vessel will be equal to the sum of the partial pressure of the individual components.
Ptotal = PA + PB
Question 24.
Give the reason behind the lowering of vapour pressure in the dissolution of NaCl in water?
Answer:
NaCI is a non volatile solute. When a non-volatile solute is dissolved in a pure solvent, the vapour pressure of pure solvent will decrease. In such a solution, vapour pressure of the solution will depend only on the solvent molecules as the solute is non-volatile.
![]()
Question 25.
What is the ideal solution? Give example.
Answer:
An ideal solution is a solution in which each component i.e., the solute as well as the solvent obeys Raoult’s law over the entire range of concentration.
Question 26.
What is volume percentage?
Answer:
It is defined as the volume of the component present in 100mL of the solution. If Va is a volume of solute and Vb is the volume of solvent, then
Vollume percentage of solute = \(\frac{V_{a} \times 100}{V_{a}+V_{b}}\)
Question 27.
What are colligative properties? Give example.
Answer:
The properties which do not depend on the chemical nature of the solute but depend only on the number of solute particles present in the solution are called colligative properties. e.g.,
- Relative lowering of vapour pressure – \(\frac { P° – P}{ P° }\)
- Osmotic pressure – π
- Elevation of the boiling point – ∆Tb
- Depression in freezing point – ∆Tf
Question 28.
What is meant by elevation of boiling point?
Answer:
1. The boiling point of a liquid is the temperature at which its vapour pressure becomes equal to the atmospheric pressure.
2. When a non-volatile solute is added to pure solvent at its boiling point, the vapour pressure of the solution is lowered below 1 atm. To bring the vapour pressure again to 1 atm, the temperature of the solution has to be increased.
3. As a result, the solution boils at a higher temperature (Tb) than the boiling point of pure solvent (Tb°). This increase in the boiling point is known as the elevation of boiling point.
Question 29.
Define ebullioscopic constant.
Answer:
Ebullioscopic constant kb, is equal to the elevation in boiling point for 1 molal solution.

Question 30.
What is Van’t Hoff factor?
Answer:
It is defined as the ratio of the actual molar mass to the abnormal (calculated) molar mass of the solute. Here, the abnormal molar mass is the molar mass calculated using the experimentally determined colligative property.
i = \(\frac{\text { Normal (actual) molar mass }}{\text { obseved (abnormal) molar mass }}\)
![]()
Question 31.
Write the Van’t Hoff equation of osmotic pressure.
Answer:
Van’t Hoff equation states that for dilute solutions, the osmotic pressure is directly proportional to the molar concentration of the solute and the temperature of the solution.
π = CRT
where
π = Osmotic pressure
C = concentration
T = Temperature
R = gas constant
Question 32.
Define Van’t Hoff factor.
Answer:
van’t Hoff factor (I) is defined as the ratin of the actual molar mass to the abnormal molar mass of the solute.

Question 33.
How is degree of dissociation and degree of association are related with van’t Hoff factor?
Answer:
The degree of dissociation or association can be related to van’t Hoff factor
1. using the following relationship
- αdissociation = \(\frac { i – 1 }{ n – 1 }\)
- αassociation = \(\frac { (1 – i)n }{ n – 1 }\)
where n = number of solute particles
Question 34.
Give an example of a solid solution ¡n which the solute is a gas.
Answer:
Solution of hydrogen in palladium.
Question 35.
Why the carbonated drinks are stored in a pressurized container?
Answer:
The carbonated beverages contain carbon dioxide dissolved in them. To dissolve the carbon dioxide in these drinks, the CO2 gas is bubbled through them under high pressure. These containers are sealed to maintain the pressure. When we open these containers at atmospheric pressure, the pressure of the CO2 drops to the atmospheric level, and hence bubbles of CO2 rapidly escape from the solution and show effervescence. The burst of bubbles is even more noticeable if the soda bottle in warm condition.
Question 36.
Why do gases always tend to be less soluble in liquids as the temperature is raised?
Answer:
Mostly dissolution of gases in liquid is an exothermic process. it is because of the fact that this process involves a decrease of entropy. Thus, an increase in temperature tends to push the equilibrium towards a backward direction as a result of which solubility of the gas decrease with a rise in temperature.
(Gas + Solvent \(\rightleftharpoons\) Solution + Heat)
Question 37.
Why is the freezing point depression of 0.1 M NaCl solution nearly twice that of 0.1M glucose solution?
Answer:
NaCl is an electrolyte and it dissociates completely whereas glucose being a non-electrolyte does not dissociate. Hence, the number of particles in 0.1 M NaCl solution is nearly double for NaCI solution than that for glucose solution of same molarity.
Therefore depression in freezing point being a colligative property ¡s nearly twice for NaCl solution than that for glucose solution of same molarity.
Question 38.
Why a person suffering from high blood pressure is advised to take a minimum quantity of common salt?
Answer:
Osmotic pressure is directly proportional to the concentration of solutes. Our body fluid contains a number of solutes. On taking large amount of salt, ions entering into the body fluid thereby raises the concentration of solutes. As a result, osmotic pressure increases which may rupture the blood cells.
Samacheer Kalvi 11th Chemistry Solutions 3 Marks Questions and Answers
Question 1.
What are gaseous solution? Give its various types with example
Answer:

Question 2.
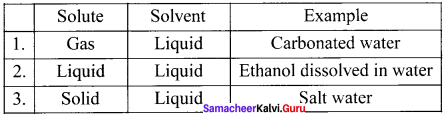
What are liquid solutions ? Explain with example
Answer:
Question 3.
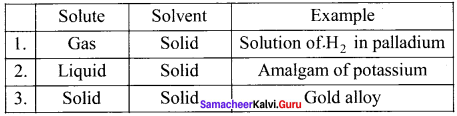
What are solid solution? Give example.
Answer:
Question 4.
How will you prepare a standard solution?
Answer:
- A standard solution or a stock solution is a solution whose concentration is accurately known.
- A standard solution of required concentration can be prepared by dissolving a required amount of a solute in a suitable amount of solvent.
- It is done by transforming a known amount of solute to a standard flask of definite volume. A small amount of water is added lo the flask and shaken well to dissolve the salt.
- Then water is added to the flask to bring the solution level lo the mark indicated at the top end of the flask.
- The flask is stoppered and shaken well to make concentration uniform.
![]()
Question 5.
What are the advantages of standard solution.
Answer:
1. The error due to weighing the solute can be minimised by using concentrated stock solution that requires large quantities of solute.
2. We can prepare working standards of different concentrations by diluting the stock solution which is more efficient since consistency is maintained.
3. Some of the concentrated solutions are more stable and are less likely to support microbial growth than working standards used in the experiments.
Question 6.
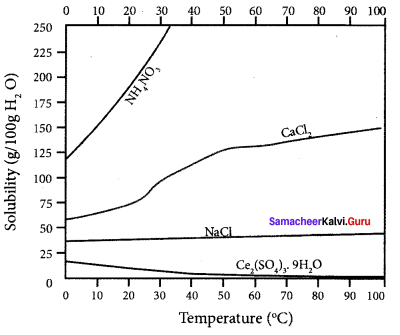
Explain the solubilities of ammonium nitrate, calcium chloride, ceric sulphate and sodium chloride in water at different temperature with a graph.
Answer:
1. The solubility of sodium chloride does not vary appreciably as the maximum solubility is achieved at normal temperature. In fact, there is only 10% increase in solubility between 0°C to 100°C.
2. The dissolution process of ammonium nitrate is endothermic, the solubility increases with
3. In the case of eerie sulphate. the dissolution is exothermic and the solubility decreases with increase in temperature.
4. Even though the dissolution of calcium chloride is exothermic, the solubility increases moderately with increase in temperature. Here the entropy factor also plays a significant role in deciding the position of equilibrium.
Question 7.
Explain the effect of temperature gaseous solute ¡n liquid solvent.
Answer:
1. In the case of gaseous solute in liquid solvent, the solubility decreases with increase in temperature.
2. When a gaseous solute dissolves in a liquid solvent, its molecules interact with solvent molecules with weak inter molecular forces when the temperature increases, the average. kinetic energy of the molecules present in the solution also increases.
3. The increase in kinetic energy breaks (he weak inter molecular forces between the gaseous solute and liquid solvent with results in the release of the dissolved gas molecules to gaseous state.
4. The dissolution of most of the gases in Liquid solvents is an endothermic process, the increase in temperature decreases the dissolution of gaseous molecules.
Question 8.
Give reason why aquatic species are less sustained in hot water?
Answer:
There will be decrease in solubility of gases in solution with increase in temperature. During summer, in hot water rivers, due to high temperature. the availability of dissolved oxygen decreases. So the aquatic species are less sustained in hot water.
Question 9.
Deep – sea divers use air diluted with helium gas in their tanks. Why? (or) Justify this statement.
Answer:
1. Deep-sea divers carry a compressed air tank for breathing at high pressure under water. This air tank contains nitrogen and oxygen which are not very soluble in blood and other body fluids at normal pressure.
2. As the pressure at the depth is far greater than the surface atmospheric pressure, more nitrogen dissolves in the blood when the diver breathes from tank.
3. When the divers ascends to the surface, the pressure decreases, the dissolved nitrogen comes out of the blood quickly forming bubbles in the blood stream.
These bubbles restrict blood flow, affect the transmission of nerve impulses and can even burst the capillaries or block them. This condition is called “the bends” which are painful and dangerous to life.
4. To avoid such dangerous condition they use air diluted with helium gas (11.7 % helium, 56.2% nitrogen and 32.1% oxygen) of lower solubility of helium in the blood than nitrogen.
Question 10.
What are the limitations of Henry’s law?
Answer:
- Henry’s law is applicable at moderate temperature and pressure only.
- Only the less soLuble gases obey Henry’s law.
- The gases reacting with solvent do not obey Henry’s law.
- The gases obeying Henrys law should not be associated or dissociated while dissolving in the solvent.
Question 11.
Explain how benzene in toluene obeys Raoult’s law.
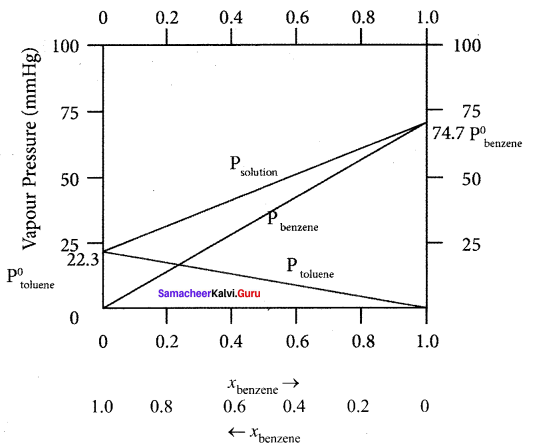
Answer:
The variation of vapour pressure of pure benzene and toluenc with its mole fraction is given in the graph.
1. The vapour pressure of pure toluene and pure benzene are 22.3 and 74.7 mm Hg respectively.
2. The graph shows the partial vapour pressure of pure components increases linearly with the increase of the mole fraction of the respective components. The total pressure at any composition of the solute and solvent is given by the straight line.
3. Psolution = P0toluene + xbenzene (P0benzene – P0toluene)
Question 12.
Derive the relationship between the relative lowering of vapour pressure and mole fraction of the solute.
Answer:
Psolution ∝ xA by Raoult’s law. where xA is the mole fraction of the solvent.
Psolution = k . xA
When
xA = 1
k = P0solvent
P0solvent = partial pressure of pure solvent
Psolution = P0solvent . xA
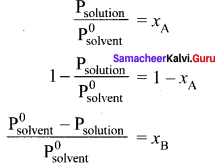
where
xB = mole fraction of the solute
xA + xB = 1
xB = 1 – xA
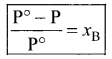
Question 13.
The depression in freezing point is 0.24 K obtained by dissolving 1 g NaCl in 200g water. Calculate van’t – Hoff factor. The molar depression constant in 1.86 K Kg mol-1
Solution :
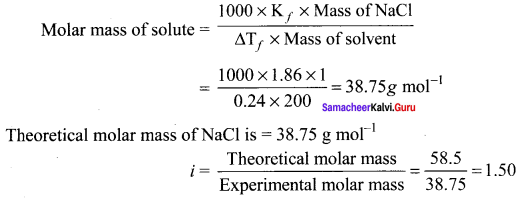
Molar mass of solute = \(\frac{1000 \times K_{f} \times \text { mass of } N a C l}{\Delta T_{f} \times \text { mass of solvent }}\)
= \(\frac{1000 \times 1.86 \times 1}{0.24 \times 200}\)
= 38.75 g mol-1
Theoretical molar mass of NaCl is = 58.5 g mol-1
i = \(\frac{\text { Theoretical molar mass }}{\text { Experimental molar mass }}\)
= \(\frac{58.5}{38.75}\) = 1.50
Question 14.
What are the necessary conditions for an ideal solution? Give two examples. For an ideal solution
1. There is no change in volume on mixing two components (solute and solvent)
∆Vmixing = O
2. There is no exchange of heat when the solute is dissolved in solvent (∆Hmixing = 0)
3. Escaping tendency of the solute and the solvent present in it should be the same as in pure liquids.
4. Examples – For ideal solution: Benzene and toluene, n-Hexane and n-Heptane, ethyl bromide and ethyl iodide, chlorobenzene and bromobenzene.
![]()
Question 15.
Explain how non-ideal solutions show positive deviation from Raoult’s law.
Answer:
- Let us consider the positive deviation shown by a solution of ethyl alcohol and water.
- In this solution, the hydrogen bonding interaction between ethanol and water is weaker than those hydrogen bonding interactions amongst themselves (ethyl alcohol-ethyl alcohol and water-water interaction).
- This results in the increased evaporation of both components from the aqueous solution of ethanol.
- Consequently, the vapour pressure of the solution is greater than the vapour pressure predicted by Raoult’s law.
- Here, the mixing OCCSS is endothermic i.e., (∆Hmixing > O) and there will be a slight increase in volume (∆Vmixing > O)
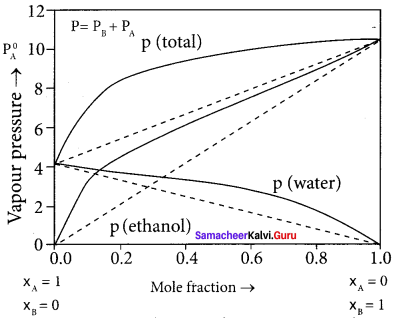
Question 16.
Explain with suitable example about negative deviation from law.
Answer:
1. Let us consider a solution of phenol and aniline. Both phenol and aniline form hydrogen-bonding interactions amongst themselves.
2. When mixed with aniline, the phenol molecule forms hydrogen bonding interactions with aniline, which are Stronger than the hydrogen bonds formed amongst themselves.
3. Formation of new hydrogen bonds considerably reduces the escaping tendency of phenol and aniline from the solution.
4. Asa result, the vapour pressure of the solution is less and there is a slight decrease in volume (∆Vmixing < 0) on mixing.
5. During this process evolution of heat takes place i.e., ∆Vmixing < 0 (exothermic).
6. Examples – Acetone + Chloroform, Chloroform + Diethyl ether
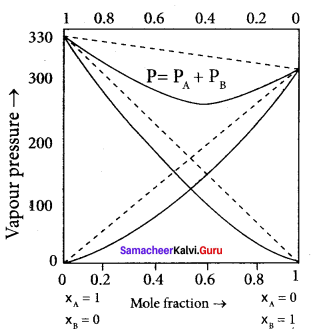
Question 17.
The vapour pressure of a solution containing a nonvolatile, non-electrolyte solute is always lower than that of pure solvent. Give reason.
Answer:
1. The vapour pressure of a solution (P) containing a full volatile solute is lower than that of pure solvent (P°).
2. Consider a closed system is which a pure solvent is in equilibrium with its vapour. At equilibrium the molar Gibbs free energies of solvent in a liquid and gaseous phase are equal (∆G = O).
3. When a solute is added to this solvent the dissolution takes place and its free energy (G) decreases due to increase in entropy.
4. In order to maintain the equilibrium, the free energy of the vapour phase must also decrease.
5. At a given temperature, the only way to lower the free energy of the vapour is to reduce its pressure.
6. Thus the vapour pressure of the solution must decrease to maintain the equilibrium.
![]()
Question 18.
Show that relative lowering of vapour pressure is a colligative property.
Answer:
According to Raoult’s law,
Psolution xA, where xA = mole fraction of solvent.
Psolution = k . xA, where k = proportionality constant
For a pure solvent,
Vapour pressure = P°, xA = 1
P°solution = k x 1 = k
Substituting P°solvent in Raoult’s law
Psolution = P°solvent . xA
Relative lowenng of vapour pressure

substituting Psolution as P°xB in the above eaquation
Relative lowering of vapour pressure
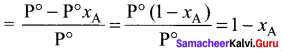
xA + xB = I
where xB = mole fraction of solute. It is clear that the relative lowering of vapour pressure depends only on the mole fraction ofthe solute (xB) and is independent of its nature. Therefore relative lowering of vapour pressure is a colligative property.
Question 19.
Explain why boiling point of solution is greater than that of pure solvent?
Answer:
When a non volatile solute is added to a pure solvent at its boiling point, the vapour pressure of the solution is lowered below 1 atm. To bring the vapour pressure again to I atm the temperature of the solution has to be increased.
As a result, the solution boils at a higher temperature (Tb) than the boiling point of the pure solvent (T°b). This increase in the boiling point is known as elevation of boiling point ∆Tb = Tb – T°b.
Question 20.
Graphically prove that Tb ¡s greater than T°b.
Answer:
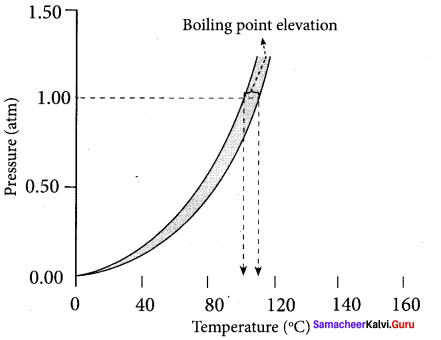
1. The vapour pressure of the solution increases with increase in temperature. The variation of vapour pressure with respect to temperature of pure water is given by the curve – A.
2. At 100°C, the vapour pressure of water is equal to I atm. Hence, the boiling point of water is 100°C (T°b).
3. When a solute is added to water, the vapour pressure of the resultant solution is lowered. The variation of vapour pressure with respect to temperature for the solution is given by curve-B.
4. From the graph, it is evident that the vapour pressure of the solution is equal to 1 atm. pressure at the temperature Tb which is greater than T°b. The difference between these two temperatures (Tb – T°b) gives the elevation of boiling point.
∆Tb = Tb T°b.
Question 21.
Derive the relationship between the elevation of boiling point and molar mass of non volatile solute.
Answer:
The elevation of boiling point ∆Tb = Tb T°b.
∆Tb is directly proportional to the concentration of the solute particles.
∆Tb ∝ m, (m = molaLiiy)
∆Tb = kb. m, where kb = ebullioscopic constant
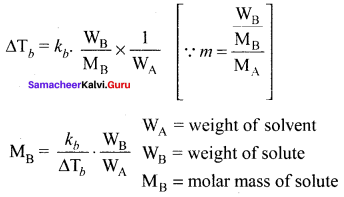
Question 22.
Define
- freezing point
- Depression in freezing point.
Explain with graph.
Answer:
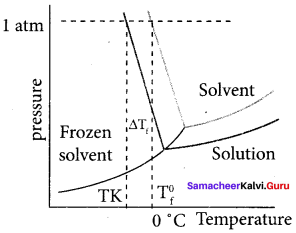
1. Freezing point is defined as the temperature at which the solid and the liquid states of the substances have the same vapour pressure.
2. When a non volatile solute is added to water at its freezing point, the freezing point of water is lowered from 0°C. The lowering of freezing point of the solvent when a solute is added is called depression in freezing point AT1..
3. ∆Tf = T0f – Tf
Question 23.
Define
- cryoscopic constant
- ebullioscopic constant
Answer:
1. ∆Tf = kf. m, where kf = cryoscopic constant. If m = 1. then ATf = kf
kf is defined as depression in freezing point for 1 molal solution.
2. ∆Tf = kf. m where kf ebullioscopic constant. If m = 1 then ATf = kf
kb is defined as elevation in boiling point for 1 molal solution.
Question 24.
What are the significances of osmotic pressure over other colligative properties ?
Answer:
1. Unlike elevation of boiling point and the depression in freezing point, the magnitude of osmotic pressure is large.
2. The osmotic pressure can be measured at room temperature enables to determine the molecular mass of biomolecules which are unstable at higher temperature.
3. Even for a very dilute solution, the osmotic pressure is large.
![]()
Question 25.
What is haemolysis ? intravenous fluid are isotonic to blood?
Answer:
1. The osmotic pressure of the blood cells is approximately equal to 7 atm at 37°C.
2. The intravenous injections should have saine osmotic pressure as that of the blood (isotonic vith blood).
3. If the intravenous solutions are too dilute that is hypotonie, the solvent from outside of the cells flow into the cell to normalise the osmotic pressure and this process is called haernolysis causes the cells to burst.
4. On the other hand, if the solution is too concentrated, that is hypertonic. the solvent molecules will flow out of the cells,which causes the cells to shrink and die.
5. For this reason, the intravenous fluids are prepared such that they are isotonic to blood (0.9% mass/volume sodium chloride solution).
Question 26.
Explain reverse osmosis.
Answer:
1. The pure water moves through the semipermeable membrane to the NaCl solution due to osmosis.
2. This process can be reversed by applying pressure greater than the osmotic pressure to the solution side. Now the pure water moves from the solution side to the solvent side and this process is called reverse osmosis.
3. Reverse osmosis can be defined as a process in which a solvent passes through a semipermeable membrane in the opposite direction of osmosis, when subjected to a hydrostatic pressure greater than the osmotic pressure.
Question 27.
Explain about the application of reverse osmosis in water purification.
Answer:
1. Reverse osmosis is used in the desalination of sea water and also in the purification of drinking water.
2. When a pressure higher than the osmotic pressure is applied on the solution side (sea water) the water molecules moves from solution side to the solvent side through semi permeable membrane (opposite to osmotic flow). The pure water can be collected.
3. Cellulose acetate (or) polyamide membranes are commonly used in commercial system.
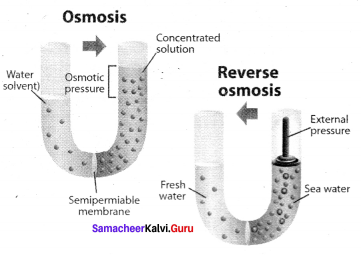
Question 28.
Acetic acid is found to have molar mass as 120 g mol-1. Prove it.
Answer:
1. In certain solvent, solute molecules associate to form a dimer. This reduces the total number of molecules formed in solution and as a result the calculated molar mass will be higher than the actual molar mass.
2. Acetic acid in benzene exist as a dimer
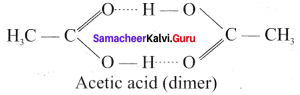
3. The molar mass of acetic acid calculate using colligative properties is found to be around 120 g mol-1 is two times of the actual molar mass 60 g mol-1.
Question 29.
Depression in freezing point of NaCI is twice that of in urea. Why?
Answer:
1. The electrolyte NaCI dissociates completely into its constituent ions in their aqueous solution. This causes an increase in the total number olparticles present in the solution.
2. When we dissolve 1 mole of NaCI in water it. dissociates and gives 1 mole of Na+ and 1 mole of Cl–. Hence the solution will have 2 moles of particles.
But when we dissolve 1 mole of urea (non electrolyte) in water it appears as 1 mole only. So the colligative property value would be double in NaCl than in urea.
![]()
Question 30.
What is van’t Hoff factor? Calculate the van’t Hoff factor value for
- acetic acid
- NaCl
Answer:
1. van’t Hoff factor is defined as the ratio ofthe actual molar mass to the abnormal (calculated) molar mass of the solute.
2.

van’t Hoff factor (1) for acetic acid = \(\frac { 60 }{ 120 }\) = 0.5
3. van’t Hoff factor (2) for NaCl = \(\frac { 117 }{ 58.5 }\) = 2
Question 31.
Differentiate between ideal solution and non-ideal solution.
Answer:
Ideal solution
An ideal solution is a solution in which each component obeys the Raoult’s law over the entire range of concentration.
For an ideal solution,
- ∆Hmixing = 0
- ∆Vmixing = 0
Example: Benzenc and toluene n – Hexane and n – Heptane
Non-ideal solution
The solutions which do not obey Raoult’slaw over the entire range of concentrationsare called non-ideal solution.
For a non-ideal solution.
- ∆Hmixing \(\quad \neq\) 0
- ∆Vmixing \(\quad \neq\) 0
Example: Ethyl alcohol and Cyclohexane, Benzene and acetone.
Question 32.
Explain the factors when i = 1, i < 1 and i >1 ?
Answer:
1. For a solute that does not dissociate or associate the vant’s hoff factor is equal to 1 (i = 1) and the molar mass will be close to the actual molar mass.
2. For that solute that associate to form higher oligomers in solution, the van’t Hoff factor will be less than 1 (i < 1) and the observed molar mass will be greater than the actual molar mass.
3. For solutes that dissociates into their constituent ions the van’t Hoff factor will be more than one (i > 1) and the observed molar mass will be less than the normal molar mass.
![]()
Question 33.
State Henry’s law and mention some of its important applications.
Answer:
Henry’s law: The solubility of a gas in a liquid is directly proportional to the pressure of the gas.
Application of Henry’s law:
- In the production of carbonated beverages
(as solubility of CO2 increase at high pressure). - In the deep sea diving.
- In the function of lungs.
- For climbers or people living at high altitudes,
Question 34.
What type of non – idealities are exhibited by cyclohexane – ethanol and acetone – chloroform mixture? Give reason for your answer.
Answer:
Ideal solutions are those which obey Raoult’s law over extreme range of concentration. Ideal solutions have another important properties:
- ∆Hmix = 0
- ∆Vmix = 0
Here-forces of attraction between A – A. B – B and A – B are of the same order. Non ideal solutions do not obey Raoult’s law over the entire range of concentration.
∆Hmixing\(\quad \neq\) 0 and ∆Vmixing\(\quad \neq\) 0
Cyclohexane – ethanol mixture shows positive deviation from Raoult’s law because forces of attraction between cyclohexane and ethanol are less than in between pure cyclohexane as well as pure ethanol.
Acetone-Chloroform mixture shows negative deviation from Raoults law because forces of attraction between acetone and chloroform are higher than that in between pure acetone and pure chloroform molecules.
![]()
Question 35.
Given below is the sketch of a plant for carrying out a process.
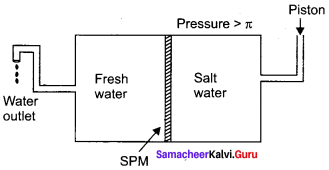
- Name the process occurring in the above plant.
- To which container does the net flow of solvent take place?
- Name one SPM which can he used in this plant.
- Give one practical use of the plant.
Answer:
- Reverse osmosis
- In fresh water container from salt water container.
- Cellulose acetate is semipermeable membrane (SPM)
- Purification of water
![]()
Question 36.
Define the term osmotic pressure. Describe how the molecular mass of a substance can be determined by a method based on measurement of osmotic pressure?
Answer:
π = CRT
π = \(\frac { n }{ V }\)RT
πV = nRT

Osmotic pressure is inversely proportional to the molecular mass of the soLute.
Question 37.
1. Menthol is a crystalline substance with peppermint taste. A 6.2% solution of menthol in cyclohexane freezes at – 1.95°C.
Determine the formula mass of menthol. The freezing point and molal depression constant of cyclohexane are 6.5°C and 20.2 K m-1, respectively.
2. State Henry’s Law and mention its two important applications.
3. Which of the following has higher boiling point and why? 0.1 M NaCl or 0.1 M Glucose
Answer:
1.

MB = 158 g mol-1
2. Henry’s Law:
The solubility of a gas in a liquid is directly proportional to the pressure of the gas.
Applications:
- Solubility of CO2 is increased at high pressure.
- Mixture of He and O2 are used by deep sea divers because he is less soluble than nitrogen.
3. 0.1 M NaCI, because it dissociates in solution and furnishes greater number of particles per unit volume while glucose being a non-electrolyte does not dissociate.
![]()
Question 38.
Water is a universal solvent. But alcohol also dissolves most of the substances soluble in water and also many more. Boiling point of water is 100°C and that of alcohol is 80°C. The specific heat of water is much higher than the specific heat of alcohol.
- List out three possible differences if instead of water as the liquid ¡n our body we had alcohol.
- What value can you derive from this special property of water and its innumerable uses in sustaining life on earth?
Answer:
1.
(i) Even a small rise in temperature in the surroundings will raise the temperature of’ the body because the specific heat of alcoholis much less than the specific heat of water. So, in order to cool the body, more sweating will take place.
(ii) As there is less H bonding in alcohol, it will gel evaporated faster. The alcohol will be evaporated at such a faster rate that the liquid has to be ingested all the time.
(iii) Ice which floats on water helps aquatic life to exist even in winter as water insulates the heat from liquid below it to go back to the surroundings. Solid alcohol does not have such special properties.
2. Praise is to the almighty that has so thoughtfully given such special properties to water and made it a liquid that could sustain life on earth.
Question 39.
State Henry’s law for solubility of a gas in a liquid. Explain the significance of Henry’s law constant (KH). At the same temperature, hydrogen is more soluble in water than helium. Which of theni will have a higher value of KH and Why?
Answer:
Henry’s law states that the solubility of a gas in liquid at a given temperature is directly proportional to the partial pressure of the gas.
P = KH x
where P is the pressure of the gas, x is the mole fraction of the gas in the solution and KH is the Henry’s law constant. KH is a function of the nature oIgas.
Higher the value of KH at a given temperature. lower is the solubility of the gas in the liquid. As helium is less soluble in water, so it has a higher value of KH than hydrogen.
Henry’s Law:
As dissolution of agar in liquid is an exothermic process, therefore, the solubility should decrease with in increase in temperature.
Question 40.
What is meant by positive and negative deviations from Raoult’s law and how is the sign ∆Hmix of related to positive and negative deviations from Raoult’s law?
Answer:
Negative deviations:
In these type of deviations, the partial vapour pressure of each component A and B of solution is higher than the vapour pressure calculated from Raoult’s law. For example -Water and ethanol, chloroform and water.
Positive deviations:
In case of positive deviation A – B interactions are weaker than those between A – A or B – B. This means that in such solutions molecules or A (or B) will find it easier to positive deviation from Raoult’s law.
Samacheer Kalvi 11th Chemistry Solutions 5 Marks Questions and Answers
II. Answer the following questions in detail:
Question 1.
- Define solution.
- Explain the types of solutions with suitable example.
Answer:
1. A solution is a homogeneous mixture of two or more substances, consisting of atoms. ions or molecules.
2. Types and examples of solution.
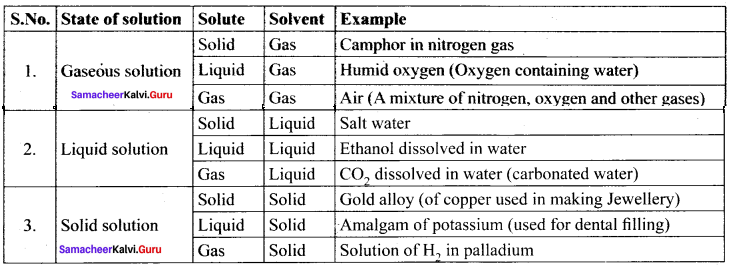
Question 2.
- Define Solubility
- Explain the factors that influences the solubility
Answer:
1. The solubility of a substance at a given temperature is defined as the amount of the solute that can be dissolved in 100 g of the solvent at a given temperature to form a saturated solution.
2. Factors influencing solubility
(a) Nature of solute and solvent: Sodium chloride, an ionic compound readily dissolves in polar solvent such as water but it does not dissolve in non polar solvent such as benzene. Most of the organic compounds dissolve in organic solvent and do not dissolve in water.
(b) Effect of temperature: Generally, the solubility of a solid solute in a liquid solvent increases with increase in temperature. The dissolution of NaCl does not vary as the maximum solubility is achieved at normal temperature.
The dissolution of ammonium nitrate is endothermic, the solubility increases with increase in temperature. The dissolution of eerie sulphate is exothermic and the solubility decreases with increase of temperature. In the case of gaseous solute in liquid solvent the soluhility decreases with increase in temperature.
Effect of pressure:
Generally the change in pressure does not have any significant effect in the solubility of solids and l?quids as they are not compressible. However, the solubility of gases generally increases with increase of pressure.
Question 3.
Explain about the factors that are responsible for deviation from Raoult’s law.
Answer:
1. Solute-solvent interactions:
For an ideal solution, the interaction between the solvent molecules (A – A), the solute molecules (B – B) and between the solvent and solute molecules (A – B) are expected to be similar. if these interactions are dissimilar, there will be a deviation from ideal behaviour.
2. Dissolution of solute:
When a solute present in a solution dissociates to give its constituent ions, the resultant ions interact strongly with the solvent and causes deviation from Raoult’s law. e.g., KCI in water deviates from ideal behaviour due to dissociation as K+ and Cl– ion which form strong ion-dipole interaction with water molecules.
3. Association of solute:
Association of solute molecules can also cause deviation from ideal behaviour. For example in solution acetic acid exists as a dimer by forming intermolecular hydrogen bonds and hence deviates from Raoult’s law.
4. Temperature:
An increase in temperature of the solution increases the average kinetic energy of the molecules present in the solution which cause decrease in the attractive force between them. As result, the solution deviates from Raoult’s law.
5. Pressure:
At high pressure, the molecules tends to stay close to each other and therefore there will be an increase in their intermolecular attraction. Thus a solution deviates from Raoult’s law at high pressure.
6. Concentration:
When the concentration is increased by adding solute, the solvent-solute interaction becomes significant. This causes deviation from Raoult’s law.
![]()
Question 4.
How would you determine molar mass from relative lowering of vapour pressure.
Answer:
1. The measurement of relative lowering of vapour pressure can be used to determine the molar mass of a non-volatile solute.
2. A known mass of the solute is dissolved in a known quantity of solvent. The relative lowering of vapour pressure is measured experimentally.
3. According to Raoult’s law, the relative lowering of vapour pressure is

WA = weight of solvent
WB = weight of solute
MA = Molar mass of solvent
MB = molar mass of solute
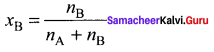
where nA = number of moles of solvent
nB = number of moles of solute.
For dilute solution, nA > > nB, nA + nB = nA
Then, ![]()
Number of moles of solvent and solute arc
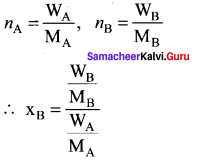
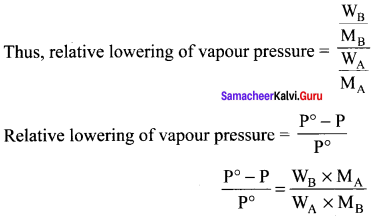
From the above equation, molar mass of the solute MB can be calculated using the known values of WA, WB, MA and the measured relative lowering of vapour pressure.
![]()
Question 5.
How would you determine the molar mass from osmotic pressure.
Answer:
According to van’t Hoff equation
π = CRT
C = \(\frac { n }{ V }\)
Here n = number of moles of solute dissolved in ‘V’ litre of the solution.
π = \(\frac { n }{ V }\) . RT = πV = nRT
If the solution is prepared by dissolving WB of the non-volatile solute in WA g of solvent, then the number of moles of ‘n’ is
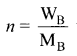
where
MB = molar mass of the solute
Substituting n value, we get
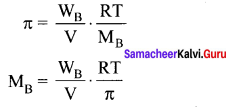
From the above equation, we can calculate the molar mass of the solute.
Question 6.
What are ideal and non-ideal solutions? Explain with suitable diagram the behaviour of ideal solutions.
Answer:
Ideal solutions:
The solutions which obey Raoult’s law over the entire range of concentration are known as ideal solutions. Ideal solutions are formed by mixing the two components which are identical in molecular size, in structure and have almost identical intermolecular forces.
Examples:
- Benzene and toluene
- n – Hexane and n-Heptane
- Chiorobenzene and bromobenzene.
Characteristics:
- They must obey Raoult’s law.
- ∆H mixing should be zero.
- ∆W mixing should be zero, i.e. volume change on mixing is zero.
Non – ideal solutions:
The solutions which do not obey Raoult’s law are called non-ideal solutions. In case of non – ideal solutions there is a change in volume and heat energy when the two components are mixed.
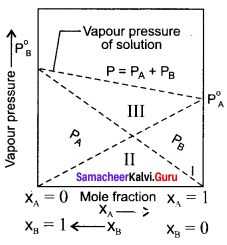
Characteristics:
- They does not obey Raoult’s law.
- ∆Vmix \(\quad \neq\) 0
- ∆Hmix \(\quad \neq\) 0
Behaviour of Ideal Solutions:
A plot of P1 or P2 versus the mole fraction x1 and x2 for an ideal solution gives a linear plot. These Lines (I and II)pass through the points and respectively whenx1 and x2 is equal to unity.
Similarly the plot (Line III) of Ptotal versus x2 is also linear. The minimum value of is P1° and the maximum value is P2°, assuming that component 1 is less volatile than component 2, i.e. P1° < P20.
![]()
Question 7.
Explain with a suitable diagram and appropriate example, why some non-ideal solution shows positive deviation from Raou It’s law.
Answer:
Some non-ideal solutions show positive deviation from Raoult’s law. Consider a solution of two components A and B. If A-B interactions in the solution are weaker than the A – A and B – B interactions in the two liquids forming the solution, then the escaping tendency of molecules A and B from the solution become more than in pure liquids.
The total vapour pressure will be greater than the corresponding vapour pressure as expected on the basis of Raoult’s law. This type of behaviour of solution is called positive deviation from Raoult’s law. The boiling point of such solutions are lowered. Mathematically,
PA < PA0 xA
PB < PB0 xB
The total vapour pressure is less than PA + PB
P < PA + PB
P < P°A x xA + P°B xB
Hence
P1 = PA
P2 = PB
Examples of solutions showing positive deviations
- Ethyl alcohol and water
- Benzene and acetone
- Ethyl alcohol and cyclohcxanc
- Carbon tetrachloride and chloroform.
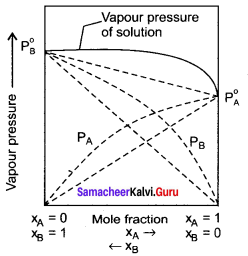
Question 8.
- What is Osmotic pressure and how is it related to the molecular mass of a non volatile substances?
- What advantage the osmotic pressure method has over the elevation of boiling point method for determining the molecular mass?
Answer:
1. Osmotic pressure:
It is the pressure of the solution column that can prevent the entry of solvent molecules through a semi-permeable membrane, when the solution and the solvent are separated by the same. It is denoted by π. Its unit is mm 11g or atmosphere.
We know that, π = CRT
where π is the osmotic pressure and R is the gas constant
π = \(\frac { { n }_{ 2 } }{ V }\) RT
where V is volume of solution per litre containing n2 moles of solute.
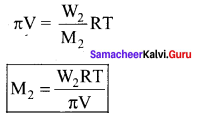
By the above relation molar mass of solute can be calculated.
2. The osmotic pressure method has the advantage over other methods as pressure measurement is around the room temperature and molarity of the solution is used instead of molality.
The technique of osmotic pressure for determination of molar mass of solutes is particularly useful for biomolecules as they are generally not stable at higher temperature and polymers have poor solubility.
III. Numerical Problems
Question 1.
Calculate the mole fraction of benzene ¡n solution containing 30% by mass in carbon tetrachioride.
Solution:
30% of benzene in carbon tetrachloride by mass means that Mass of benzene in the solution = 30g
Mass of solution = 100g
Mass of carbon tetrachloride = 100g – 30g = 70g
Molar mass of benzene (C6H6) = 78 g mol-1
Molar mass of CCl4 = 12 + (4 x 35.5) 154g mol-1
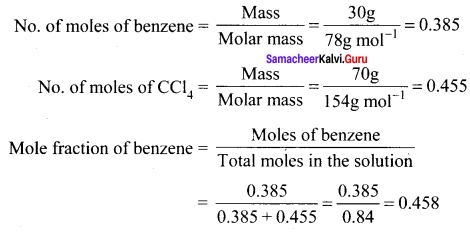
Question 2.
Calculate (he molarity of each of the following solutions:
Solution:
- 30 g of CO(NO3)2. 6H2O = in 4.3 L of solution
- 30 mL of 0.5 M H2SO4 diluted to 500 mL.
1. Molar mass of CO(NO3)2. 6H2O = 58.7 + 2(14 + 48) + (6 x 8)g mol-1
= 58.7 + 124 + 108g mol-1 = 290.7 gmol-1
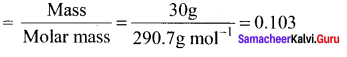
Volume of solution = 4.3 L
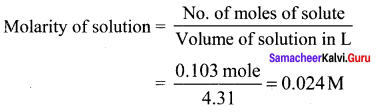
2. 1000 mL of 0.5 M H2SO4 Contain H2SO4 = 0.5 moles
30 mL of 0.5 M H2SO4 contain H2SO4 = \(\frac { 0.5 }{ 1000 }\) x 30 mole = 0.015 mole
Volume of solution = 500 mL = 0.500 L

Question 3.
Calculate the mass of urea (NH2CONH2) required in making 2.5 kg of 0.25 molal aqueous solution.
Solution:
0.25 molal aqueous solution means that
Moles of urea = 0.25 mole
Mass of solvent (water) = 1 kg = 1000 g
Molar mass of urea = 14 + 2 + 12 + 16 + 14 + 2 = 6Og mol-1
0.25 mole of urea = 60 x 0.25 mole = 15 g
Total mass of the solution = 1000 + 15 g = 1015 g = 1.015 g
Thus, 1.015 kg of solution contain urea = 15 g
2.5 kg of solution will require urea = \(\frac { 15 }{ 1.015 }\) x 2.5 kg = 37g
![]()
Question 4.
H2S, a toxic gas with rotten egg like smell is used for the qualitative analysis. 1f the solubility of H2S in water at STP is 0.195 m. Calculate Henrvs law constant.
Solution:
Solubility of H2S gas = 0.195 m
= 0.195 mole in 1 kg of the Solvent (water)
1 kg of the solvent (water) = 1000 g 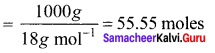
Mole fraction of H2S gas in the solution (x) = \(\frac { 0.195 }{ 0.195 + 55.55 }\) = 0.0035
Pressure at STP = 0.98 7 bar
Applying Henry’s law
PH2S = KH x xH2S
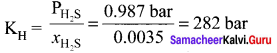
Question 5.
Vapour pressure of pure water at 298 K is 23.8 mm Hg. 50 g of urea (NH2CONH2) is dissolved in 850 g of water. Calculate the vapour pressure of water for this solution and its relative lowering.
Solution:
Here
P°1 = 23.8 mm
W2 = 50g
M2 (urea) = 60g mol-1
W1 = 850g
M1(Water) = 18g mol-1
Here we have to calculate Ps
Applying Raoult’s law,
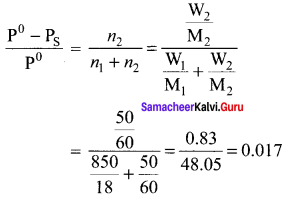
Thus, relative lowering of vapour pressure = 0.017
Substituting P° = 23.8 mm Hg
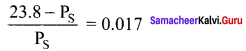
We get,
23.8 – Ps = 0.017 Ps
Ps = 23.4 mm Hg
Thus, vapour pressure of water in the solution = 23.4 mm Hg
![]()
Question 6.
Boiling point of water at 750 mm Hg is 99.63°C. How much sucrose is to be added to 500g of water such that it boils at 100°C? .
Solution:
Elevation in boiling point required, ∆Tb = 100 – 99.63° = 0.37°
Mass of solvent (water) W1 = 500g
Mass of solute, C12H22O11 = 342 g mol-1
Molar mass of solvent M1 = 18 g mol-1
Applying the formula,

Question 7.
Concentrated nitric acid used in laboratory work is 68% nitric acid by mass in aqueous solution. What should be the molarity of such a sample of the acid if the density of the solution is 1.504g mL-1?
Solution:
68% nitric acid by mass means that
Mass of nitric acid = 68 g
Mass of solution = 100 g
Molar mass of HNO3 = 63g mol-1
68g HNO3 = mole = 1.079 mole
Density of solution = 1.504 g mL-1
Volume of solution = \(\frac { 100 }{ 1.504 }\) mL = 66.5 mL = 0.0665 L
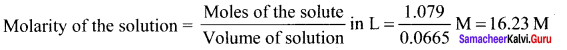
Question 8.
A solution is obtained by mixing 300 g of 25% solution and 400 g of 40% solution by mass. Calculate tile mass percentage of the resulting solution.
Solution:
300 g of 25% solution contains solute = 75g
400g of 40% solution contains solute = 160 g
Total mass of solute = 160 + 75 = 235 g
Total mass oÍ’ solution = 300 + 400 = 700 g
% of solute in the final solution = \(\frac { 235 }{ 700 }\) x 1oo = 33.5%
% of water in the finaI solution = 100 – 33.5 = 66.5%
![]()
Question 9.
A sample of drinking water was found to be severely contaminated with chloroform (CHCI3) supposed to be a carcinogen. The level of contamination was 15 ppm (by mass)
- express this in percentage by mass
- determine the molality of chloroform in the water sample.
Solution:
1. 15 ppm means 15 parts in million (106) parts by mass in the solution
% of mass = \(\frac { 15 }{ { 10 }^{ 6 } }\) x 100 = 1.5 x 10-4
2. Taking 15g chloroform in 106g of the solution
Mass of the solvent = 106 g
Molar mass of CHCl3 = 12 + 1 + (3 x 35.5) = 119.5 g mol-1
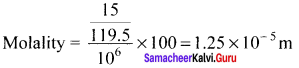
Question 10.
An aqueous solution of 2% non-volatile solute exerts a pressure of 1.004 bar at normal boiling point of the solvent. What is the molar mass of the solute?
Solution:
Vapour pressure of pure water at the boiling point
(P°) = 1 atm 1.013 bar
Vapour pressure of solution Ps = 1.004 bar
M1 = 18 g mol-1
M2 = ?
Mass of solute = W2 = 2g
Mass of solution = 100g
Mass of solvent W1 = 98 g
Applying Raoult’s law for dilute solution
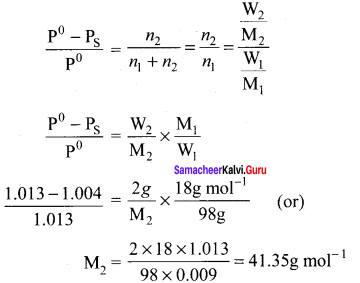
Question 11.
A 5% solution (by mass) of cane sugar in water has freezing point of 271 K. Calculate the freezing point of 5% glucose in water if freezing point of pure water is 273.15K.
Solution:
Molar mass of cane sugar
C12H22O11 = 342 g mol-1
Molality of sugar = \(\frac { 5 x 1000 }{ 342 x 100 }\) = 0.146
∆T2 for sugar solution = 273.15 – 271 = 2.15°
∆Tf = Kf x m
Kf = \(\frac { 2.15 }{ 0.146 }\)
Molality of glucose solution = \(\frac { 5 }{ 180 }\) x \(\frac { 1000 }{ 100 }\) = 0.278 m
∆Tf (Glucose) = \(\frac { 2.15 }{ 0.146 }\) x 0.278 = 4.09°K
Freezing point of glucose solution = 273.15 – 4.09 = 269.06 K
![]()
Question 12.
Calculate the amount of benzoic acid (C6HCOOH) required for preparing 250 mL of 0.15 M solution in methanol.
Solution:
0.15 M solution means that 0.15 moles of C6H5COOH is present in IL
= 1000 mL of the solution
Molar mass of C6H5COOH = 72 + 5 + 12 + 32 + 1 = 122 g mol-1
Thus, 1000 mL of solution contains benzoic acid = 18.3g
250 mL of solution will contain benzoic acid
= \(\frac { 18.3 }{ 1000 }\) x 250 = 4.575 g
Question 13.
A solution containing 8 g of a substance in 100 g of diethyl ether boils at 36.86°C, whereas pure ether boils at 35.60 °C. Determine the molecular mass of the solute (For ether Kb 2.02 K kg mol-1)
Solution:
We have, mass of solute, W2 = 8 g
Mass of solvent, W1 = 100 g
Elevation of boiling point
∆Tb = 36.86 – 35.60 = 1.260C
Kb = 2.02
Molecular mass of the solute
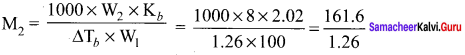
= 128.25g mol-1
Question 14.
Ethylene glycol (molar mass = 62 g mol-1) is a common automobile antifreeze. Calculate the freezing point of a solution containing 12.4 of this substance in 100 g of water. Would it be advisable to keep this substance ¡n the car radiator during summer? (Kf for water = 1.86 k kg/mol-1) (Kb for water = 0.512 K kg/mol-1)
Solution:
![]()
Since water boils at 100°C, so a solution containing ethylene glycol will boil at 101.024 °C, SO it is advisable to keep this substance in car radiator during summer.
Question 15.
15.0 g of an unknown molecular material is dissolved in 450g of water. The resulting solution freezes at – 0.34°C. What is the molar mass of the material? Kf for water = 1.86 K kg mol-1.
Solution:
W(solute) = 1 5.0 g
W(solvent) = 450 g
∆Tf = T°f – Tf = 0 – ( – 0.34) = 0.34 °C
∆Tf = kf m
0.34 = 1.86 x \(\frac { 15 }{ M }\) x \(\frac { 1000 }{ 450 }\)
M = \(\frac { 1.86 x 15 x 1000 }{ 0.34 x 450 }\) = 182.35 g mol-1
![]()
Common Errors
Common Errors:
- Students are writing solute, solvent, students get confused to write A (or) B, 1 (or)2.
- Mole, mole fraction may be confused.
- In writing osmosis definition Students get confused in mentioning concentration terms.
- van’t Hoff factor I formula may be con fused.
- Students may get contused rhen they write solute and solvent.
- Mole and mole fraction may be confused by students.
- Standard solutions must be known.
- When students write Raoult’s law, they get confused with solute and solvent.
- When they write the definition of osmosis, the conc. term may be little con fused.
- van’t Hoff equation may be written wrongly.
Rectifications:
- Always solvent is first so it is denoted as A (or) 1 solute is second so, it is denoted as B (or)2.
- Mole = n ; Mole fraction x
- Osmosis-movenient of solvent from low concentration to high concentration through a semipermeable membrane.

- For e.g.. solid in liquid means solid is the solute and liquid is the solvent.
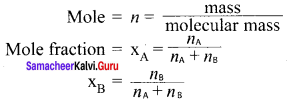
- 1 N = 1 normal solution, 0.1 N Decinormal solution, 0.01 N = Centinormal solution
- In Raoult’s law, “A” is always solvent and “B” is always solute.
- In osmosis, always solvent rnoes through semi permeable membrane from low concentration to high concentration.
- van’t Hoff factory = i